Using induced pluripotent stem cells derived neurons to model brain diseases
- PMID: 28852383
- PMCID: PMC5558480
- DOI: 10.4103/1673-5374.211180
Using induced pluripotent stem cells derived neurons to model brain diseases
Abstract
The ability to use induced pluripotent stem cells (iPSC) to model brain diseases is a powerful tool for unraveling mechanistic alterations in these disorders. Rodent models of brain diseases have spurred understanding of pathology but the concern arises that they may not recapitulate the full spectrum of neuron disruptions associated with human neuropathology. iPSC derived neurons, or other neural cell types, provide the ability to access pathology in cells derived directly from a patient's blood sample or skin biopsy where availability of brain tissue is limiting. Thus, utilization of iPSC to study brain diseases provides an unlimited resource for disease modelling but may also be used for drug screening for effective therapies and may potentially be used to regenerate aged or damaged cells in the future. Many brain diseases across the spectrum of neurodevelopment, neurodegenerative and neuropsychiatric are being approached by iPSC models. The goal of an iPSC based disease model is to identify a cellular phenotype that discriminates the disease-bearing cells from the control cells. In this mini-review, the importance of iPSC cell models validated for pluripotency, germline competency and function assessments is discussed. Selected examples for the variety of brain diseases that are being approached by iPSC technology to discover or establish the molecular basis of the neuropathology are discussed.
Keywords: brain diseases; induced pluripotent stem cells; molecular mechanisms; neuron cell models; therapeutics; translational medicine.
Conflict of interest statement
Conflicts of interest: None declared.
Figures
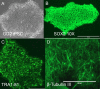
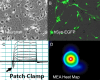
References
-
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao FB. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol. 2013;126:385–399. - PMC - PubMed
-
- Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol. 2011;7:603–615. - PubMed
-
- Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, Gorris M, Galet B, Palomares V, Brown J, Bang AG, Mertens J, Bohnke L, Boyer L, Simon S, Gage FH. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc Natl Acad Sci U S A. 2015;112:E2725–2734. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

