Activity of anthracycline- and ifosfamide-based chemotherapy in a series of patients affected by advanced myxofibrosarcoma
- PMID: 28852467
- PMCID: PMC5568720
- DOI: 10.1186/s13569-017-0082-6
Activity of anthracycline- and ifosfamide-based chemotherapy in a series of patients affected by advanced myxofibrosarcoma
Abstract
Background: We report on the activity of anthracycline-based and high-dose prolonged-infusion ifosfamide chemotherapy in a retrospective series of patients affected by advanced myxofibrosarcoma treated at Istituto Nazionale Tumori in Milan, Italy, and within the Italian Rare Cancer Network (RTR).
Methods: Advanced myxofibrosarcoma patients treated with anthracycline + ifosfamide and high-dose prolonged-infusion ifosfamide as a single agent from November 2001 to December 2016 were retrospectively reviewed. All pathological diagnosis were centrally reviewed by at least two expert pathologists. Response was evaluated by RECIST, and survival functions were computed.
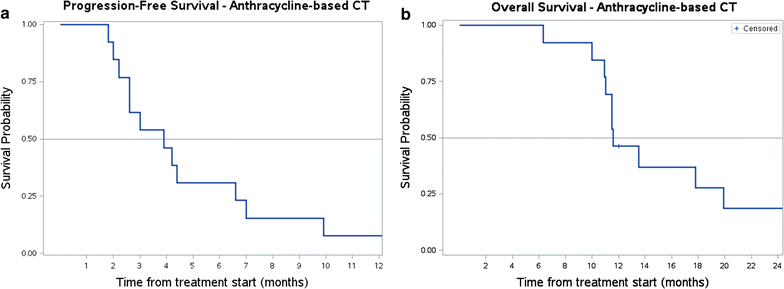
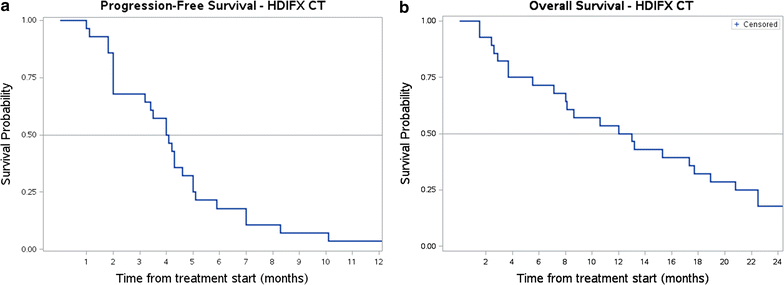
Results: Among 34 advanced myxofibrosarcoma patients, 13 were treated with front-line anthracycline + ifosfamide chemotherapy (male/female = 6/7, median age 54 years, range 33-72). Overall best response was: 4 partial responses, 3 stable diseases and 6 progressive diseases, with a median progression-free survival of 4 months. Twenty-eight patients received second/further line high-dose prolonged-infusion ifosfamide (male/female = 17/11, median age 55 years, range 27-75 years). We observed 10 partial responses, 4 stable diseases and 14 progressive diseases, with a median progression-free survival of 4 months. Median overall survival was 12 months.
Conclusions: This retrospective analysis suggests that the combination of anthracyclines and ifosfamide is active in myxofibrosarcoma. In patients already treated with a combination of anthracyclines and ifosfamide, high-dose prolonged-infusion ifosfamide showed activity as well.
Keywords: Chemotherapy; High-dose prolonged-infusion ifosfamide; Myxofibrosarcoma; Soft tissue sarcoma.
Figures

References
-
- Angervall L, Kindblom LG, Merck C. Myxofibrosarcoma. A study of 30 cases. Acta Pathol Microbiol Scand. 1977;85A:127–140. - PubMed
-
- Fletcher CDM, Bridge JA, Pancras CW, Mertens F, editors. World Health Organization (WHO) classification of tumours of soft tissue and bone. Pathology and Genetics. Lyon: IARC Press; 2013.
-
- Merck C, Angervall L, Kindblom LG, Odén A. Myxofibrosarcoma. A malignant soft tissue tumor of fibroblastichistiocytic origin. A clinicopathologic and prognostic study of 110 cases using multivariate analysis. Acta Pathol Microbiol Immunol Scand Suppl. 1983;282:1–40. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

