Bisphenol A Impairs Synaptic Plasticity by Both Pre- and Postsynaptic Mechanisms
- PMID: 28852612
- PMCID: PMC5566242
- DOI: 10.1002/advs.201600493
Bisphenol A Impairs Synaptic Plasticity by Both Pre- and Postsynaptic Mechanisms
Abstract
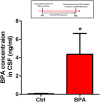
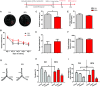
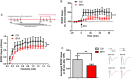
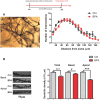
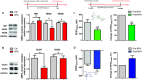
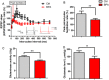
Bisphenol A (BPA), an environmental xenoestrogen, has been reported to induce learning and memory impairments in rodent animals. However, effects of BPA exposure on synaptic plasticity and the underlying physiological mechanisms remain elusive. Our behavioral and electrophysiological analyses show that BPA obviously perturbs hippocampal spatial memory of juvenile Sprague-Dawley rats after four weeks exposure, with significantly impaired long-term potentiation (LTP) in the hippocampus. These effects involve decreased spine density of pyramidal neurons, especially the apical dendritic spine. Further presynaptic findings show an overt inhibition of pulse-paired facilitation during electrophysiological recording, which suggest the decrease of presynaptic transmitter release and is consistent with reduced production of presynaptic glutamate after BPA exposure. Meanwhile, LTP-related glutamate receptors, NMDA receptor 2A (NR2A) and AMPA receptor 1 (GluR1), are significantly downregulated in BPA-exposed rats. Excitatory postsynaptic currents (EPSCs) results also show that EPSCNMDA, but not EPSCAMPA, is declined by 40% compared to the baseline in BPA-perfused brain slices. Taken together, these findings reveal that juvenile BPA exposure has negative effects on synaptic plasticity, which result from decreases in dendritic spine density and excitatory synaptic transmission. Importantly, this study also provides new insights into the dynamics of BPA-induced memory deterioration during the whole life of rats.
Keywords: bisphenol A; hippocampus; spatial memory; spine; synaptic plasticity.
Figures
Similar articles
-
Lead Exposure Impairs Hippocampus Related Learning and Memory by Altering Synaptic Plasticity and Morphology During Juvenile Period.Mol Neurobiol. 2016 Aug;53(6):3740-3752. doi: 10.1007/s12035-015-9312-1. Epub 2015 Jul 4. Mol Neurobiol. 2016. PMID: 26141123
-
Bisphenol A exposure remodels cognition of male rats attributable to excitatory alterations in the hippocampus and visual cortex.Toxicology. 2018 Dec 1;410:132-141. doi: 10.1016/j.tox.2018.10.002. Epub 2018 Oct 9. Toxicology. 2018. PMID: 30312744
-
Early developmental bisphenol-A exposure sex-independently impairs spatial memory by remodeling hippocampal dendritic architecture and synaptic transmission in rats.Sci Rep. 2016 Aug 31;6:32492. doi: 10.1038/srep32492. Sci Rep. 2016. PMID: 27578147 Free PMC article.
-
LTD, LTP, and the sliding threshold for long-term synaptic plasticity.Hippocampus. 1996;6(1):35-42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. Hippocampus. 1996. PMID: 8878740 Review.
-
Expression mechanisms underlying NMDA receptor-dependent long-term potentiation.Ann N Y Acad Sci. 1999 Apr 30;868:515-25. doi: 10.1111/j.1749-6632.1999.tb11320.x. Ann N Y Acad Sci. 1999. PMID: 10414328 Review.
Cited by
-
Protective effect of crocin on bisphenol A - induced spatial learning and memory impairment in adult male rats: Role of oxidative stress and AMPA receptor.Iran J Basic Med Sci. 2020 Sep;23(9):1146-1154. doi: 10.22038/ijbms.2020.41097.9714. Iran J Basic Med Sci. 2020. PMID: 32963736 Free PMC article.
-
Transgenerational Bisphenol A Causes Deficits in Social Recognition and Alters Postsynaptic Density Genes in Mice.Endocrinology. 2019 Aug 1;160(8):1854-1867. doi: 10.1210/en.2019-00196. Endocrinology. 2019. PMID: 31188430 Free PMC article.
-
Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors.Front Mol Neurosci. 2018 Aug 29;11:307. doi: 10.3389/fnmol.2018.00307. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30210294 Free PMC article. Review.
-
Recording from an Identified Neuron Efficiently Reveals Hazard for Brain Function in Risk Assessment.Molecules. 2021 Nov 17;26(22):6935. doi: 10.3390/molecules26226935. Molecules. 2021. PMID: 34834026 Free PMC article. Review.
-
Neuro-toxic and Reproductive Effects of BPA.Curr Neuropharmacol. 2019;17(12):1109-1132. doi: 10.2174/1570159X17666190726112101. Curr Neuropharmacol. 2019. PMID: 31362658 Free PMC article. Review.
References
-
- a) Cao X. L., Corriveau J., Popovic S., J. Agric. Food Chem. 2009, 57, 1307; - PubMed
- b) Geens T., Roosens L., Neels H., Covaci A., Chemosphere 2009, 76, 755; - PubMed
- c) Xu J., Wu L., Chen W., Chang A. C., J. Chromatogr. A 2008, 1202, 189; - PubMed
- d) Lorber M., Schecter A., Paepke O., Shropshire W., Christensen K., Birnbaum L., Environ. Int. 2015, 77, 55; - PMC - PubMed
- e) Wang W., Abualnaja K. O., Asimakopoulos A. G., Covaci A., Gevao B., Johnson‐Restrepo B., Kumosani T. A., Malarvannan G., Minh T. B., Moon H. B., Nakata H., Sinha R. K., Kannan K., Environ. Int. 2015, 83, 183. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
