Diketopyrrolopyrrole-Based Conjugated Polymer Entailing Triethylene Glycols as Side Chains with High Thin-Film Charge Mobility without Post-Treatments
- PMID: 28852623
- PMCID: PMC5566237
- DOI: 10.1002/advs.201700048
Diketopyrrolopyrrole-Based Conjugated Polymer Entailing Triethylene Glycols as Side Chains with High Thin-Film Charge Mobility without Post-Treatments
Abstract
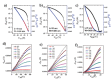
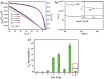
Side chain engineering of conjugated donor-acceptor polymers is a new way to manipulate their optoelectronic properties. Two new diketopyrrolopyrrole (DPP)-terthiophene-based conjugated polymers PDPP3T-1 and PDPP3T-2, with both hydrophilic triethylene glycol (TEG) and hydrophobic alkyl chains, are reported. It is demonstrated that the incorporation of TEG chains has a significant effect on the interchain packing and thin-film morphology with noticeable effect on charge transport. Polymer chains of PDPP3T-1 in which TEG chains are uniformly distributed can self-assemble spontaneously into a more ordered thin film. As a result, the thin film of PDPP3T-1 exhibits high saturated hole mobility up to 2.6 cm2 V-1 s-1 without any post-treatment. This is superior to those of PDPP3T with just alkyl chains and PDPP3T-2. Moreover, the respective field effect transistors made of PDPP3T-1 can be utilized for sensing ethanol vapor with high sensitivity (down to 100 ppb) and good selectivity.
Keywords: charge carrier mobility; conjugated donor–acceptor polymer; diketopyrrolopyrrole; side chains; triethylene glycol.
Figures



Similar articles
-
Modification of Side Chains of Conjugated Molecules and Polymers for Charge Mobility Enhancement and Sensing Functionality.Acc Chem Res. 2018 Jun 19;51(6):1422-1432. doi: 10.1021/acs.accounts.8b00069. Epub 2018 May 17. Acc Chem Res. 2018. PMID: 29771491
-
Use of side-chain for rational design of n-type diketopyrrolopyrrole-based conjugated polymers: what did we find out?Phys Chem Chem Phys. 2014 Aug 28;16(32):17253-65. doi: 10.1039/c4cp02322f. Phys Chem Chem Phys. 2014. PMID: 25017861
-
Influence of Branched Alkyl Ester-Labeled Side Chains on Specific Chain Arrangement and Charge-Transport Properties of Diketopyrrolopyrrole-Based Conjugated Polymers.ACS Appl Mater Interfaces. 2018 Nov 28;10(47):40681-40691. doi: 10.1021/acsami.8b13292. Epub 2018 Nov 13. ACS Appl Mater Interfaces. 2018. PMID: 30381941
-
Rational design of benzotrithiophene-diketopyrrolopyrrole-containing donor-acceptor polymers for improved charge carrier transport.Adv Mater. 2013 Oct 11;25(38):5467-72. doi: 10.1002/adma.201302052. Epub 2013 Jul 21. Adv Mater. 2013. PMID: 23873786
-
The Effects of Side Chains on the Charge Mobilities and Functionalities of Semiconducting Conjugated Polymers beyond Solubilities.Adv Mater. 2019 Nov;31(46):e1903104. doi: 10.1002/adma.201903104. Epub 2019 Sep 4. Adv Mater. 2019. PMID: 31483542 Review.
Cited by
-
Controlling n-Type Molecular Doping via Regiochemistry and Polarity of Pendant Groups on Low Band Gap Donor-Acceptor Copolymers.Macromolecules. 2021 Apr 27;54(8):3886-3896. doi: 10.1021/acs.macromol.1c00317. Epub 2021 Apr 8. Macromolecules. 2021. PMID: 34054145 Free PMC article.
-
Unveiling the Role of Side Chain for Improving Nonvolatile Characteristics of Conjugated Polymers-Based Artificial Synapse.Adv Sci (Weinh). 2024 Apr;11(16):e2400304. doi: 10.1002/advs.202400304. Epub 2024 Feb 26. Adv Sci (Weinh). 2024. PMID: 38408158 Free PMC article.
-
Electrode and dielectric layer interface device engineering study using furan flanked diketopyrrolopyrrole-dithienothiophene polymer based organic transistors.Sci Rep. 2020 Nov 17;10(1):19989. doi: 10.1038/s41598-020-76962-x. Sci Rep. 2020. PMID: 33203904 Free PMC article.
-
Preparation and Electrochromic Properties of Benzodithiophene-Isoindigo Conjugated Polymers with Oligoethylene Glycol Side Chains.Materials (Basel). 2022 Dec 21;16(1):60. doi: 10.3390/ma16010060. Materials (Basel). 2022. PMID: 36614403 Free PMC article.
-
Elucidating Design Rules toward Enhanced Solid-State Charge Transport in Oligoether-Functionalized Dioxythiophene-Based Alternating Copolymers.ACS Appl Mater Interfaces. 2023 Jul 26;15(29):35227-35238. doi: 10.1021/acsami.3c00053. Epub 2023 Jul 14. ACS Appl Mater Interfaces. 2023. PMID: 37449957 Free PMC article.
References
-
- Zaumseil J., Sirringhaus H., Chem. Rev. 2007, 107, 1296. - PubMed
-
- Lei T., Wang J. Y., Pei J., Acc. Chem. Res. 2014, 47, 1117. - PubMed
-
- Li Y., Acc. Chem. Res. 2012, 45, 723. - PubMed
-
- Liu Z., Zhang G., Cai Z., Chen X., Luo H., Li Y., Wang J., Zhang D., Adv. Mater. 2014, 26, 6965. - PubMed
-
- Guo X., Facchetti A., Marks T. J., Chem. Rev. 2014, 114, 8943. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
