Comparison of Standardized Cytomegalovirus (CMV) Viral Load Thresholds in Whole Blood and Plasma of Solid Organ and Hematopoietic Stem Cell Transplant Recipients with CMV Infection and Disease
- PMID: 28852681
- PMCID: PMC5570102
- DOI: 10.1093/ofid/ofx143
Comparison of Standardized Cytomegalovirus (CMV) Viral Load Thresholds in Whole Blood and Plasma of Solid Organ and Hematopoietic Stem Cell Transplant Recipients with CMV Infection and Disease
Abstract
Background: Quantification of cytomegalovirus (CMV) deoxyribonucleic acid (DNA) has important diagnostic, prognostic, and therapeutic implications in the management of transplant recipients. We aimed to assess a viral load in plasma and whole blood that distinguishes CMV disease from asymptomatic infection in a cohort of solid organ and hematopoietic stem cell transplantation.
Methods: We prospectively measured and compared CMV viral load in paired plasma and whole blood samples collected from transplant recipients with CMV infection and disease. Cytomegalovirus viral loads were determined by a commercially available US Food and Drug Administration-approved quantitative assay (COBAS AmpliPrep/COBAS TaqMan CMV Test [CAP/CTM CMV]) calibrated to the first World Health Organization International Standard for CMV DNA quantification.
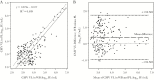
Results: Moderate agreement of CMV viral load was observed between plasma and whole blood, with 31% of samples having discordant findings, particularly among samples with low DNA levels. Among the subset of samples where both paired samples had quantifiable levels, we observed a systematic bias that reflected higher viral load in whole blood compared with plasma. Based on receiver operating curve analysis, an initial plasma CMV viral load threshold of 1700 IU/mL in solid organ transplant recipients (sensitivity 80%, specificity 74%) and 1350 IU/mL in allogeneic hematopoietic stem cell transplant recipients (sensitivity 87%, specificity 87%) distinguished CMV disease and asymptomatic infection.
Conclusions: This study identifies standardized viral load thresholds that distinguish CMV disease from asymptomatic infection using CAP/CTM CMV assay. We propose these thresholds as potential triggers to be evaluated in prospective studies of preemptive therapy. Plasma was better than whole blood for measuring viral load using the CAP/CTM CMV assay.
Keywords: CMV DNA; cytomegalovirus; transplantation; viral load.
Figures

Similar articles
-
Evaluation of COBAS AmpliPrep/COBAS TaqMan CMV Test for use in hematopoietic stem cell transplant recipients.Expert Rev Mol Diagn. 2017 Jul;17(7):633-639. doi: 10.1080/14737159.2017.1325737. Epub 2017 May 15. Expert Rev Mol Diagn. 2017. PMID: 28468570 Review.
-
CMV antigenemia and quantitative viral load assessments in hematopoietic stem cell transplant recipients.J Clin Virol. 2013 Feb;56(2):108-12. doi: 10.1016/j.jcv.2012.10.001. Epub 2012 Nov 10. J Clin Virol. 2013. PMID: 23146665
-
Prospective comparison of cytomegalovirus quantification in whole blood and plasma samples among hematopoietic stem cell transplant and kidney transplant recipients.J Clin Virol. 2024 Oct;174:105690. doi: 10.1016/j.jcv.2024.105690. Epub 2024 May 13. J Clin Virol. 2024. PMID: 38852538
-
Monitoring of cytomegalovirus viral loads by two molecular assays in whole-blood and plasma samples from hematopoietic stem cell transplant recipients.J Clin Microbiol. 2015 Apr;53(4):1252-7. doi: 10.1128/JCM.03435-14. Epub 2015 Feb 4. J Clin Microbiol. 2015. PMID: 25653404 Free PMC article.
-
Clinical utility of cytomegalovirus viral load in solid organ transplant recipients.Curr Opin Infect Dis. 2015 Aug;28(4):317-22. doi: 10.1097/QCO.0000000000000173. Curr Opin Infect Dis. 2015. PMID: 26098497 Review.
Cited by
-
Cytomegalovirus Pneumonia in a Patient with Down Syndrome.Medicina (Kaunas). 2024 Jan 30;60(2):242. doi: 10.3390/medicina60020242. Medicina (Kaunas). 2024. PMID: 38399530 Free PMC article.
-
A Systematic Review and Meta-analysis of Optimized CMV Preemptive Therapy and Antiviral Prophylaxis for CMV Disease Prevention in CMV High-Risk (D+R-) Kidney Transplant Recipients.Transplant Direct. 2023 Jul 12;9(8):e1514. doi: 10.1097/TXD.0000000000001514. eCollection 2023 Aug. Transplant Direct. 2023. PMID: 37456587 Free PMC article.
-
The Viral Load of Human Cytomegalovirus Infection in Children following Hematopoietic Stem Cell Transplant by Chip Digital PCR.Can J Infect Dis Med Microbiol. 2022 Oct 17;2022:2786841. doi: 10.1155/2022/2786841. eCollection 2022. Can J Infect Dis Med Microbiol. 2022. PMID: 36300166 Free PMC article.
-
The Standardization and Control of Serology and Nucleic Acid Testing for Infectious Diseases.Clin Microbiol Rev. 2021 Dec 15;34(4):e0003521. doi: 10.1128/CMR.00035-21. Epub 2021 Jul 28. Clin Microbiol Rev. 2021. PMID: 34319148 Free PMC article. Review.
-
Correlation of cytomegalovirus viral load between whole blood and plasma of congenital cytomegalovirus infection under valganciclovir treatment.BMC Infect Dis. 2023 Jan 19;23(1):31. doi: 10.1186/s12879-023-07995-6. BMC Infect Dis. 2023. PMID: 36658533 Free PMC article.
References
-
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13 (Suppl 4):93–106. - PubMed
-
- Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am 2010; 24:319–37. - PubMed
-
- Sia IG, Wilson JA, Groettum CM et al. . Cytomegalovirus (CMV) DNA load predicts relapsing CMV infection after solid organ transplantation. J Infect Dis 2000; 181:717–20. - PubMed
-
- Razonable RR, Åsberg A, Rollag H et al. . Virologic suppression measured by a cytomegalovirus (CMV) DNA test calibrated to the World Health Organization international standard is predictive of CMV disease resolution in transplant recipients. Clin Infect Dis 2013; 56:1546–53. - PubMed
-
- Caliendo AM. The long road toward standardization of viral load testing for cytomegalovirus. Clin Infect Dis 2013; 56:374–5. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

