SMN regulation in SMA and in response to stress: new paradigms and therapeutic possibilities
- PMID: 28852871
- PMCID: PMC6201753
- DOI: 10.1007/s00439-017-1835-2
SMN regulation in SMA and in response to stress: new paradigms and therapeutic possibilities
Abstract
Low levels of the survival of motor neuron (SMN) protein cause the neurodegenerative disease spinal muscular atrophy (SMA). SMA is a pediatric disease characterized by spinal motor neuron degeneration. SMA exhibits several levels of severity ranging from early antenatal fatality to only mild muscular weakness, and disease prognosis is related directly to the amount of functional SMN protein that a patient is able to express. Current therapies are being developed to increase the production of functional SMN protein; however, understanding the effect that natural stresses have on the production and function of SMN is of critical importance to ensuring that these therapies will have the greatest possible effect for patients. Research has shown that SMN, both on the mRNA and protein level, is highly affected by cellular stress. In this review we will summarize the research that highlights the roles of SMN in the disease process and the response of SMN to various environmental stresses.
Conflict of interest statement
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures
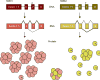
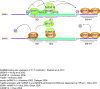

References
-
- Akten Bikem, Kye Min Jeong, Hao Le T, Wertz Mary H, Singh Sasha, Nie Duyu, Huang Jia, et al. Interaction of Survival of Motor Neuron (SMN) and HuD Proteins with mRNA Cpg15 Rescues Motor Neuron Axonal Deficits…. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(25):10337–42. doi: 10.1073/pnas.1104928108. - DOI - PMC - PubMed
-
- Alías Laura, Bernal Sara, Fuentes-Prior Pablo, Barceló María Jesus, Also Eva, Martínez-Hernández Rebeca, Rodríguez-Alvarez Francisco J, et al. Mutation Update of Spinal Muscular Atrophy in Spain: Molecular Characterization of 745 Unrelated Patients and Identification of Four Novel Mutations in the SMN1 Gene…. Human Genetics. 2009;125(1):29–39. doi: 10.1007/s00439-008-0598-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

