Compensating for cross-reactions using avidity and computation in a suspension multiplex immunoassay for serotyping of Zika versus other flavivirus infections
- PMID: 28852878
- PMCID: PMC5599479
- DOI: 10.1007/s00430-017-0517-y
Compensating for cross-reactions using avidity and computation in a suspension multiplex immunoassay for serotyping of Zika versus other flavivirus infections
Abstract
The recent spread of Zika virus (ZIKV) in the Americas and Asia necessitates an increased preparedness for improved maternal and perinatal health and blood safety. However, serological cross-reactions, especially to Dengue virus (DENV), complicate ZIKV antibody serodiagnosis. A novel "pan-Flavi" suspension multiplex immunoassay (PFSMIA) using 25 antigens, whole virus (WV), non-structural protein 1 (NS1), and envelope (E) proteins, from 7 zoonotic flaviviruses for specific detection of ZIKV and DENV IgM and IgG was developed. Patterns of antibody cross-reactivity, avidity, and kinetics were established in 104 sera from returning travelers with known ZIKV and DENV infections. PFSMIA gave IgM- and IgG-sensitivities for both viruses of 96-100%, compared to an immunofluorescence assay. Main IgM cross-reactions were to NS1, for IgG to the E and WV antigens. Infecting virus yielded reactivity to several antigens of the homologous virus, while cross-reactions tended to occur only to a single antigen from heterologous virus(es). A specificity-enhancing computer procedure took into account antibody isotype, number of antibody-reactive antigens per virus, avidity, average degree of cross-reactivity to heterologous flavivirus antigens, and reactivity changes in serial sera. It classified all 50 cases correctly. Applied to sera from 200 pregnant women and 173 blood donors from Sweden, one blood donor was found ZIKV NS1 IgM positive, and another as ZIKV NS1 IgG positive. These samples did not react with other ZIKV antigens and were thereby judged as false-positives. PFSMIA provided sensitive and specific ZIKV and DENV serology, warranting high-throughput serological surveillance and a minimized need for laborious and expensive virus neutralization assays.
Keywords: Dengue virus; Flavivirus; Pathogen surveillance; Serological cross-reaction; Suspension multiplex immunoassay; Zika virus.
Conflict of interest statement
Jonas Blomberg submitted a patent application [16] regarding enhanced specificity of flavivirus serology using computational and avidity-based compensation. The other authors do not have potential conflicts of interest.
Figures

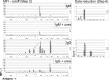


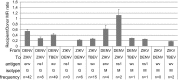


Similar articles
-
Re-evaluation of routine dengue virus serology in travelers in the era of Zika virus emergence.J Clin Virol. 2017 Jul;92:25-31. doi: 10.1016/j.jcv.2017.05.001. Epub 2017 May 3. J Clin Virol. 2017. PMID: 28505571
-
Use of the immunoglobulin G avidity assay to differentiate between recent Zika and past dengue virus infections.Clin Sci (Lond). 2019 Apr 4;133(7):859-867. doi: 10.1042/CS20180874. Print 2019 Apr 15. Clin Sci (Lond). 2019. PMID: 30902827
-
Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016.Euro Surveill. 2016 Dec 15;21(50):30426. doi: 10.2807/1560-7917.ES.2016.21.50.30426. Euro Surveill. 2016. PMID: 28006649 Free PMC article.
-
Serological cross-reactivity among common flaviviruses.Front Cell Infect Microbiol. 2022 Sep 15;12:975398. doi: 10.3389/fcimb.2022.975398. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36189346 Free PMC article. Review.
-
Humoral cross-reactivity between Zika and dengue viruses: implications for protection and pathology.Emerg Microbes Infect. 2017 May 10;6(5):e33. doi: 10.1038/emi.2017.42. Emerg Microbes Infect. 2017. PMID: 28487557 Free PMC article. Review.
Cited by
-
Emerging infectious disease laboratory and diagnostic preparedness to accelerate vaccine development.Hum Vaccin Immunother. 2019;15(10):2258-2263. doi: 10.1080/21645515.2019.1634992. Epub 2019 Jul 16. Hum Vaccin Immunother. 2019. PMID: 31268394 Free PMC article. Review.
-
Distinction between serological responses following tick-borne encephalitis virus (TBEV) infection vs vaccination, Sweden 2017.Euro Surveill. 2018 Jan;23(3):17-00838. doi: 10.2807/1560-7917.ES.2018.23.3.17-00838. Euro Surveill. 2018. PMID: 29386094 Free PMC article.
-
Immunoglobulin M seroneutralization for improved confirmation of Japanese encephalitis virus infection in a flavivirus-endemic area.Trans R Soc Trop Med Hyg. 2022 Nov 1;116(11):1032-1042. doi: 10.1093/trstmh/trac036. Trans R Soc Trop Med Hyg. 2022. PMID: 35593182 Free PMC article.
-
Serologic Tools and Strategies to Support Intervention Trials to Combat Zika Virus Infection and Disease.Trop Med Infect Dis. 2019 Apr 19;4(2):68. doi: 10.3390/tropicalmed4020068. Trop Med Infect Dis. 2019. PMID: 31010134 Free PMC article. Review.
-
Determining seropositivity-A review of approaches to define population seroprevalence when using multiplex bead assays to assess burden of tropical diseases.PLoS Negl Trop Dis. 2021 Jun 28;15(6):e0009457. doi: 10.1371/journal.pntd.0009457. eCollection 2021 Jun. PLoS Negl Trop Dis. 2021. PMID: 34181665 Free PMC article. Review.
References
-
- WHO . Zika virus expected to spread in Europe in late spring and summer: overall risk low to moderate. Copenhagen: WHO Media centre; 2016.
-
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science. 2016 - PubMed
-
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, Ahmed R, Suthar MS, Wrammert J. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci USA. 2016;113(28):7852–7857. doi: 10.1073/pnas.1607931113. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

