Metabolic liver function in humans measured by 2-18F-fluoro-2-deoxy-D-galactose PET/CT-reproducibility and clinical potential
- PMID: 28853060
- PMCID: PMC5574826
- DOI: 10.1186/s13550-017-0320-1
Metabolic liver function in humans measured by 2-18F-fluoro-2-deoxy-D-galactose PET/CT-reproducibility and clinical potential
Abstract
Background: PET/CT with the radioactively labelled galactose analogue 2-18F-fluoro-2-deoxy-D-galactose (18F-FDGal) can be used to quantify the hepatic metabolic function and visualise regional metabolic heterogeneity. We determined the day-to-day variation in humans with and without liver disease. Furthermore, we examined whether the standardised uptake value (SUV) of 18F-FDGal from static scans can substitute the hepatic systemic clearance of 18F-FDGal (K met, mL blood/min/mL liver tissue/) quantified from dynamic scans as measure of metabolic function. Four patients with cirrhosis and six healthy subjects underwent two 18F-FDGal PET/CT scans within a median interval of 15 days for determination of day-to-day variation. The correlation between K met and SUV was examined using scan data and measured arterial blood concentrations of 18F-FDGal (blood samples) from 14 subjects from previous studies. Regional and whole-liver values of K met and SUV along with total metabolic liver volume and total metabolic liver function (total SUV, average SUV multiplied by total metabolic liver volume) were calculated.
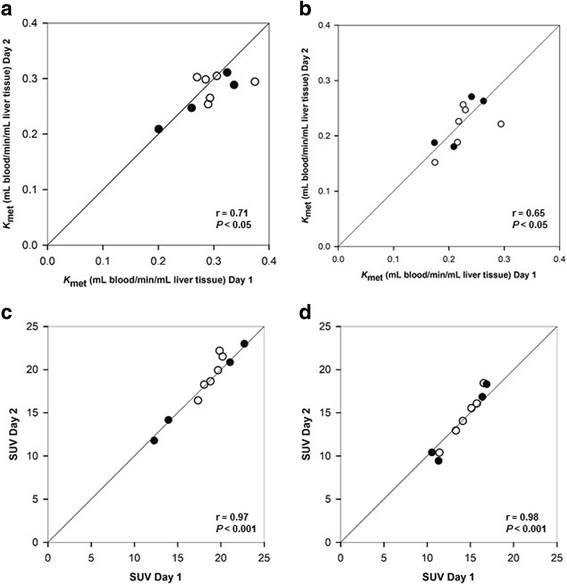
Results: No significant day-to-day differences were found for K met or SUV. SUV had higher intraclass correlation coefficients than K met (0.92-0.97 vs. 0.49-0.78). The relationship between K met and SUV was linear. Total metabolic liver volume had non-significant day-to-day variation (median difference 50 mL liver tissue; P = 0.6). Mean total SUV in healthy subjects was 23,840 (95% CI, 21,609; 26,070), significantly higher than in the patients (P < 0.001).
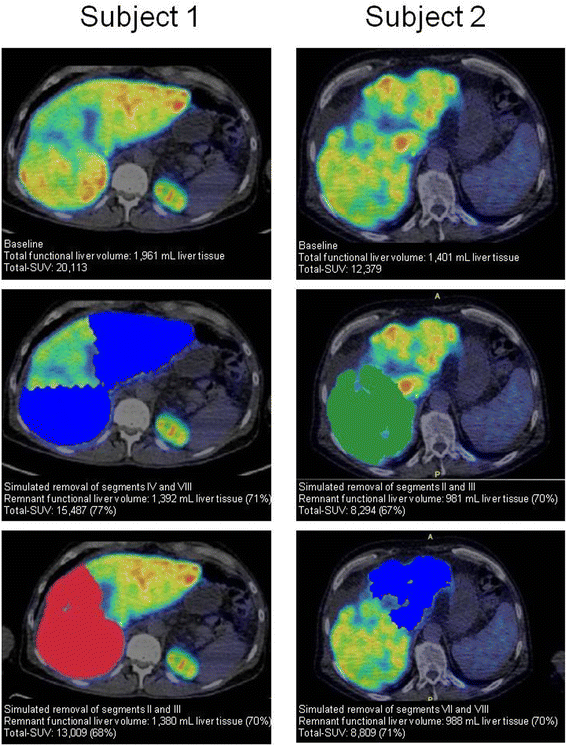
Conclusions: The reproducibility of 18F-FDGal PET/CT was good and SUV can substitute K met for quantification of hepatic metabolic function. Total SUV of 18F-FDGal is a promising tool for quantification of metabolic liver function in pre-treatment evaluation of individual patients.
Keywords: Galactose; Metabolic liver function; Molecular imaging; Positron emission tomography; Remnant liver function.
Conflict of interest statement
Ethics approval and consent to participate
All procedures were in accordance with the ethical standards of the national research committee and with the principles of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Cieslak KP, Bennink RJ, de Graaf W, van Lienden KP, Besselink MG, Busch OR, Gouma DJ, van Gulik TM. Measurement of liver function using hepatobiliary scientigraphy improves risk assessment in patients undergoing major liver resection. HPB. 2016;18:773–780. doi: 10.1016/j.hpb.2016.06.006. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

