Lung Endothelial MicroRNA-1 Regulates Tumor Growth and Angiogenesis
- PMID: 28853613
- PMCID: PMC5736970
- DOI: 10.1164/rccm.201610-2157OC
Lung Endothelial MicroRNA-1 Regulates Tumor Growth and Angiogenesis
Abstract
Rationale: Vascular endothelial growth factor down-regulates microRNA-1 (miR-1) in the lung endothelium, and endothelial cells play a critical role in tumor progression and angiogenesis.
Objectives: To examine the clinical significance of miR-1 in non-small cell lung cancer (NSCLC) and its specific role in tumor endothelium.
Methods: miR-1 levels were measured by Taqman assay. Endothelial cells were isolated by magnetic sorting. We used vascular endothelial cadherin promoter to create a vascular-specific miR-1 lentiviral vector and an inducible transgenic mouse. KRASG12D mut/Trp53-/- (KP) mice, lung-specific vascular endothelial growth factor transgenic mice, Lewis lung carcinoma xenografts, and primary endothelial cells were used to test the effects of miR-1.
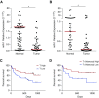
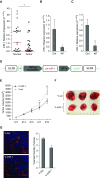
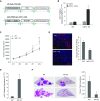
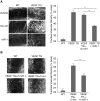
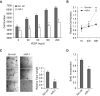
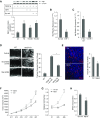
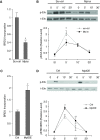
Measurements and main results: In two cohorts of patients with NSCLC, miR-1 levels were lower in tumors than the cancer-free tissue. Tumor miR-1 levels correlated with the overall survival of patients with NSCLC. miR-1 levels were also lower in endothelial cells isolated from NSCLC tumors and tumor-bearing lungs of KP mouse model. We examined the significance of lower miR-1 levels by testing the effects of vascular-specific miR-1 overexpression. Vector-mediated delivery or transgenic overexpression of miR-1 in endothelial cells decreased tumor burden in KP mice, reduced the growth and vascularity of Lewis lung carcinoma xenografts, and decreased tracheal angiogenesis in vascular endothelial growth factor transgenic mice. In endothelial cells, miR-1 level was regulated through phosphoinositide 3-kinase and specifically controlled proliferation, de novo DNA synthesis, and ERK1/2 activation. Myeloproliferative leukemia oncogene was targeted by miR-1 in the lung endothelium and regulated tumor growth and angiogenesis.
Conclusions: Endothelial miR-1 is down-regulated in NSCLC tumors and controls tumor progression and angiogenesis.
Keywords: angiogenesis; lung cancer; microRNA-1; tumor microenvironment; vascular endothelial growth factor blockers.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

