NDUFAF4 variants are associated with Leigh syndrome and cause a specific mitochondrial complex I assembly defect
- PMID: 28853723
- PMCID: PMC5643967
- DOI: 10.1038/ejhg.2017.133
NDUFAF4 variants are associated with Leigh syndrome and cause a specific mitochondrial complex I assembly defect
Abstract
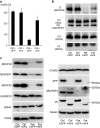
Mitochondrial respiratory chain complex I consists of 44 different subunits and can be subgrouped into three functional modules: the Q-, the P- and the N-module. NDUFAF4 (C6ORF66) is an assembly factor of complex I that associates with assembly intermediates of the Q-module. Via exome sequencing, we identified a homozygous missense variant in a complex I-deficient patient with Leigh syndrome. Supercomplex analysis in patient fibroblasts revealed specifically altered stoichiometry. Detailed assembly analysis of complex I, indicative of all of its assembly routes, showed an accumulation of parts of the P- and the N-module but not the Q-module. Lentiviral complementation of patient fibroblasts with wild-type NDUFAF4 rescued complex I deficiency and the assembly defect, confirming the causal role of the variant. Our report on the second family affected by an NDUFAF4 variant further characterizes the phenotypic spectrum and sheds light into the role of NDUFAF4 in mitochondrial complex I biogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Guerrero-Castillo S, Baertling F, Kownatzki D et al: The assembly pathway of mitochondrial respiratory chain complex I. Cell Metab 2017; 25: 128–139. - PubMed
-
- Schwarz JM, Cooper DN, Schuelke M, Seelow D: MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 2014; 11: 361–362. - PubMed
-
- Kumar P, Henikoff S, Ng PC: Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009; 4: 1073–1081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

