Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function
- PMID: 28853742
- PMCID: PMC6389332
- DOI: 10.1038/ejcn.2017.132
Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function
Abstract
Background/objectives: Glutathione (GSH) is the most abundant endogenous antioxidant and a critical regulator of oxidative stress. Maintenance of optimal tissues for GSH levels may be an important strategy for the prevention of oxidative stress-related diseases. We investigated if oral administration of liposomal GSH is effective at enhancing GSH levels in vivo.
Subjects/methods: A 1-month pilot clinical study of oral liposomal GSH administration at two doses (500 and 1000 mg of GSH per day) was conducted in healthy adults. GSH levels in whole blood, erythrocytes, plasma and peripheral blood mononuclear cells (PBMCs) were assessed in 12 subjects at the baseline and after 1, 2 and 4 weeks of GSH administration.
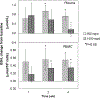
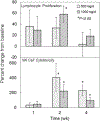
Results: GSH levels were elevated after 1 week with maximum increases of 40% in whole blood, 25% in erythrocytes, 28% in plasma and 100% in PBMCs occurring after 2 weeks (P<0.05). GSH increases were accompanied by reductions in oxidative stress biomarkers, including decreases of 35% in plasma 8-isoprostane and 20% in oxidized:reduced GSH ratios (P<0.05). Enhancements in immune function markers were observed with liposomal GSH administration including Natural killer (NK) cell cytotoxicity, which was elevated by up to 400% by 2 weeks (P<0.05), and lymphocyte proliferation, which was elevated by up to 60% after 2 weeks (P<0.05). Overall, there were no differences observed between dose groups, but statistical power was limited due to the small sample size in this study.
Conclusions: Collectively, these preliminary findings support the effectiveness of daily liposomal GSH administration at elevating stores of GSH and impacting the immune function and levels of oxidative stress.
Conflict of interest statement
Conflict of Interest:
RS and JPR received research funding for this study from Researched Nutritionals, LLC. Researched Nutritionals, LLC is a nutraceutical company that provides liposomal glutathione (Tri-Fortify™ Orange) to health care professionals. Other than providing research funding and liposomal GSH, Researched Nutritionals, LLC did not play a role in the design of the study, collection and analysis of the data and decision to publish. There were no personal financial interests between any of the authors with Researched Nutritionals, LLC.
Figures


References
-
- Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. Faseb J 1999; 13(10): 1169–1183. - PubMed
-
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983; 52: 711–760. - PubMed
-
- Vina J Glutathione: Metabolism and Physiological Functions, CRC Press: Boca Raton, 1990.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

