Implementation of a New Clinic-Based, Pharmacist-Managed PCSK9 Inhibitor Consultation Service
- PMID: 28854074
- PMCID: PMC10397962
- DOI: 10.18553/jmcp.2017.23.9.918
Implementation of a New Clinic-Based, Pharmacist-Managed PCSK9 Inhibitor Consultation Service
Abstract
Background: The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors alirocumab and evolocumab were approved by the FDA in 2015. In anticipation of provider interest and a potential increase in referrals to the on-site specialty pharmacy, we created a pharmacist-managed consultation service.
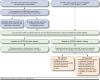
Program description: The development of a clinic-based pharmacist-managed consultation service for the management of the PCSK9 inhibitor agents alirocumab and evolocumab is described. Key implementation steps included (a) creation of a pharmacy team and collaboration with cardiology; (b) completion of a needs assessment; (c) service creation; (d) collaboration with the on-site specialty pharmacy; (e) development of an electronic consult order and consult pool; (f) personnel training; and (g) service approval and marketing. The service development occurred over 9 months (July 2015-April 2016) and was implemented hospital-wide in May 2016.
Observations: The University of Illinois Hospital and Health Sciences System PCSK9 inhibitor consultation service successfully integrated the benefits of a clinical review process, information technology capabilities of an electronic medical record system, and collaboration with the on-site specialty pharmacy to provide a comprehensive service that aimed to facilitate appropriate medication management from prescribing to patient administration and provide monitoring for this class of specialty medications.
Implications/recommendations: The PCSK9 pharmacist-managed consultation service provides a method for complex therapies to be managed comprehensively through the collaboration of ambulatory care clinics and outpatient specialty pharmacies.
Disclosures: No outside funding supported this study. Groo reports speaker bureau fees from Pfizer and Bristol-Myers Squibb. The other authors have nothing to disclose. All the authors contributed to study concept and design. Atande took the lead in data collection, and data interpretation was performed by Groo and Atanda. The manuscript was written by Atanda and revised by all the authors.
Conflict of interest statement
No outside funding supported this study. Groo reports speaker bureau fees from Pfizer and Bristol-Myers Squibb. The other authors have nothing to disclose.
All the authors contributed to study concept and design. Atande took the lead in data collection, and data interpretation was performed by Groo and Atanda. The manuscript was written by Atanda and revised by all the authors.
Figures
References
-
- PRALUENT (alirocumab) injection, for subcutaneous use. Sanofi/Regeneron. Revised April 2017. Available at: http://products.sanofi.us/praluent/praluent.pdf. Accessed July 18, 2017.
-
- REPATHA (evolocumab) injection, for subcutaneous use. Amgen. Revised July 2016. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/repatha/r.... Accessed July 18, 2017.
-
- Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489-99. - PubMed
-
- Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-09. - PubMed
-
- Roth EM, Taskinene MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized phase 3 trial. Int J Cardiol. 2014;176(1):55-61. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous