The 5'-poly(A) leader of poxvirus mRNA confers a translational advantage that can be achieved in cells with impaired cap-dependent translation
- PMID: 28854224
- PMCID: PMC5595341
- DOI: 10.1371/journal.ppat.1006602
The 5'-poly(A) leader of poxvirus mRNA confers a translational advantage that can be achieved in cells with impaired cap-dependent translation
Abstract
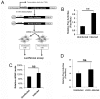
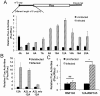
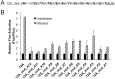
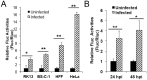
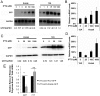
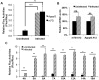
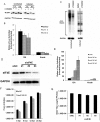
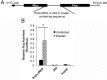
The poly(A) leader at the 5'-untranslated region (5'-UTR) is an unusually striking feature of all poxvirus mRNAs transcribed after viral DNA replication (post-replicative mRNAs). These poly(A) leaders are non-templated and of heterogeneous lengths; and their function during poxvirus infection remains a long-standing question. Here, we discovered that a 5'-poly(A) leader conferred a selective translational advantage to mRNA in poxvirus-infected cells. A constitutive and uninterrupted 5'-poly(A) leader with 12 residues was optimal. Because the most frequent lengths of the 5'-poly(A) leaders are 8-12 residues, the result suggests that the poly(A) leader has been evolutionarily optimized to boost poxvirus protein production. A 5'-poly(A) leader also could increase protein production in the bacteriophage T7 promoter-based expression system of vaccinia virus, the prototypic member of poxviruses. Interestingly, although vaccinia virus post-replicative mRNAs do have 5'- methylated guanosine caps and can use cap-dependent translation, in vaccinia virus-infected cells, mRNA with a 5'-poly(A) leader could also be efficiently translated in cells with impaired cap-dependent translation. However, the translation was not mediated through an internal ribosome entry site (IRES). These results point to a fundamental mechanism poxvirus uses to efficiently translate its post-replicative mRNAs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Poxvirus-encoded decapping enzymes promote selective translation of viral mRNAs.PLoS Pathog. 2020 Oct 8;16(10):e1008926. doi: 10.1371/journal.ppat.1008926. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33031446 Free PMC article.
-
La-related protein 4 is enriched in vaccinia virus factories and is required for efficient viral replication in primary human fibroblasts.bioRxiv [Preprint]. 2023 Mar 11:2023.03.10.532125. doi: 10.1101/2023.03.10.532125. bioRxiv. 2023. Update in: Microbiol Spectr. 2023 Aug 18:e0139023. doi: 10.1128/spectrum.01390-23. PMID: 36945573 Free PMC article. Updated. Preprint.
-
In Vitro Transcribed RNA-based Luciferase Reporter Assay to Study Translation Regulation in Poxvirus-infected Cells.J Vis Exp. 2019 May 1;(147):10.3791/59626. doi: 10.3791/59626. J Vis Exp. 2019. PMID: 31107441 Free PMC article.
-
Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation.Biol Res. 2005;38(2-3):121-46. doi: 10.4067/s0716-97602005000200003. Biol Res. 2005. PMID: 16238092 Review.
-
Ribosomes in poxvirus infection.Curr Opin Virol. 2022 Oct;56:101256. doi: 10.1016/j.coviro.2022.101256. Epub 2022 Oct 18. Curr Opin Virol. 2022. PMID: 36270183 Free PMC article. Review.
Cited by
-
Messenger RNAs of Yeast Virus-Like Elements Contain Non-templated 5' Poly(A) Leaders, and Their Expression Is Independent of eIF4E and Pab1.Front Microbiol. 2019 Oct 30;10:2366. doi: 10.3389/fmicb.2019.02366. eCollection 2019. Front Microbiol. 2019. PMID: 31736885 Free PMC article.
-
mTOR Dysregulation by Vaccinia Virus F17 Controls Multiple Processes with Varying Roles in Infection.J Virol. 2019 Jul 17;93(15):e00784-19. doi: 10.1128/JVI.00784-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31118254 Free PMC article.
-
Negative charge in the RACK1 loop broadens the translational capacity of the human ribosome.Cell Rep. 2021 Sep 7;36(10):109663. doi: 10.1016/j.celrep.2021.109663. Cell Rep. 2021. PMID: 34496247 Free PMC article.
-
The African Swine Fever Virus Transcriptome.J Virol. 2020 Apr 16;94(9):e00119-20. doi: 10.1128/JVI.00119-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32075923 Free PMC article.
-
Poxviruses Evade Cytosolic Sensing through Disruption of an mTORC1-mTORC2 Regulatory Circuit.Cell. 2018 Aug 23;174(5):1143-1157.e17. doi: 10.1016/j.cell.2018.06.053. Epub 2018 Aug 2. Cell. 2018. PMID: 30078703 Free PMC article.
References
-
- Walsh D, Mathews MB, Mohr I. Tinkering with translation: protein synthesis in virus-infected cells. Cold Spring Harbor Perspect Biol. 2013;5(1):a012351 doi: 10.1101/cshperspect.a012351 . - DOI - PMC - PubMed
-
- Walsh D, Mohr I. Viral subversion of the host protein synthesis machinery. Nat Rev Microbiol. 2011;9(12):860–75. doi: 10.1038/nrmicro2655 . - DOI - PMC - PubMed
-
- Cao S, Dhungel P, Yang Z. Going against the Tide: Selective Cellular Protein Synthesis during Virally Induced Host Shutoff. J Virol. 2017;91(17). doi: 10.1128/JVI.00071-17 . - DOI - PMC - PubMed
-
- Yang Z, Martens CA, Bruno DP, Porcella SF, Moss B. Pervasive initiation and 3'-end formation of poxvirus postreplicative RNAs. J Biol Chem. 2012;287(37):31050–60. doi: 10.1074/jbc.M112.390054 . - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

