Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus
- PMID: 28854233
- PMCID: PMC5595347
- DOI: 10.1371/journal.ppat.1006601
Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus
Abstract
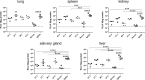
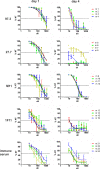
Human cytomegalovirus (HCMV) is an important, ubiquitous pathogen that causes severe clinical disease in immunocompromised individuals, such as organ transplant recipients and infants infected in utero. Antiviral chemotherapy remains problematic due to toxicity of the available compounds and the emergence of viruses resistant to available antiviral therapies. Antiviral antibodies could represent a valuable alternative strategy to limit the clinical consequences of viral disease in patients. The envelope glycoprotein B (gB) of HCMV is a major antigen for the induction of virus neutralizing antibodies. However, the role of anti-gB antibodies in the course of the infection in-vivo remains unknown. We have used a murine CMV (MCMV) model to generate and study a number of anti-gB monoclonal antibodies (mAbs) with differing virus-neutralizing capacities. The mAbs were found to bind to similar antigenic structures on MCMV gB that are represented in HCMV gB. When mAbs were used in immunodeficient RAG-/- hosts to limit an ongoing infection we observed a reduction in viral load both with mAbs having potent neutralizing capacity in-vitro as well as mAbs classified as non-neutralizing. In a therapeutic setting, neutralizing mAbs showed a greater capacity to reduce the viral burden compared to non-neutralizing antibodies. Efficacy was correlated with sustained concentration of virus neutralizing mAbs in-vivo rather than their in-vitro neutralizing capacity. Combinations of neutralizing mAbs further augmented the antiviral effect and were found to be as potent in protection as polyvalent serum from immune animals. Prophylactic administration of mAbs before infection was also protective and both neutralizing and non-neutralizing mAbs were equally effective in preventing lethal infection of immunodeficient mice. In summary, our data argue that therapeutic application of potently neutralizing mAbs against gB represent a strategy to modify the outcome of CMV infection in immunodeficient hosts. When present before infection, both neutralizing and non-neutralizing anti-gB exhibited protective capacity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Reviews in Medical Virology. 2007. pp. 253–276. doi: 10.1002/rmv.535 - DOI - PubMed
-
- Ludwig A, Hengel H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill Bull Eur sur les Mal Transm = Eur Commun Dis Bull. 2009;14: 26–32. - PubMed
-
- Dreher AM, Arora N, Fowler KB, Novak Z, Britt WJ, Boppana SB, et al. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr. 2014;164: 855–859. doi: 10.1016/j.jpeds.2013.12.007 - DOI - PMC - PubMed
-
- Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: a review. Infect Chemother. 2013;45: 260–71. doi: 10.3947/ic.2013.45.3.260 - DOI - PMC - PubMed
-
- Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: A CIBMTR analysis. Blood. 2016;127: 2427–2438. doi: 10.1182/blood-2015-11-679639 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

