Interaction of the cyclic-di-GMP binding protein FimX and the Type 4 pilus assembly ATPase promotes pilus assembly
- PMID: 28854278
- PMCID: PMC5595344
- DOI: 10.1371/journal.ppat.1006594
Interaction of the cyclic-di-GMP binding protein FimX and the Type 4 pilus assembly ATPase promotes pilus assembly
Abstract
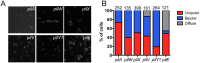
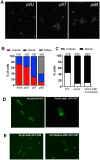
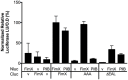
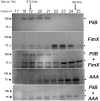
Type IVa pili (T4P) are bacterial surface structures that enable motility, adhesion, biofilm formation and virulence. T4P are assembled by nanomachines that span the bacterial cell envelope. Cycles of T4P assembly and retraction, powered by the ATPases PilB and PilT, allow bacteria to attach to and pull themselves along surfaces, so-called "twitching motility". These opposing ATPase activities must be coordinated and T4P assembly limited to one pole for bacteria to show directional movement. How this occurs is still incompletely understood. Herein, we show that the c-di-GMP binding protein FimX, which is required for T4P assembly in Pseudomonas aeruginosa, localizes to the leading pole of twitching bacteria. Polar FimX localization requires both the presence of T4P assembly machine proteins and the assembly ATPase PilB. PilB itself loses its polar localization pattern when FimX is absent. We use two different approaches to confirm that FimX and PilB interact in vivo and in vitro, and further show that point mutant alleles of FimX that do not bind c-di-GMP also do not interact with PilB. Lastly, we demonstrate that FimX positively regulates T4P assembly and twitching motility by promoting the activity of the PilB ATPase, and not by stabilizing assembled pili or by preventing PilT-mediated retraction. Mutated alleles of FimX that no longer bind c-di-GMP do not allow rapid T4P assembly in these assays. We propose that by virtue of its high-affinity for c-di-GMP, FimX can promote T4P assembly when intracellular levels of this cyclic nucleotide are low. As P. aeruginosa PilB is not itself a high-affinity c-di-GMP receptor, unlike many other assembly ATPases, FimX may play a key role in coupling T4P mediated motility and adhesion to levels of this second messenger.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
A bacterial sense of touch: T4P retraction motor as a means of surface sensing by Pseudomonas aeruginosa PA14.J Bacteriol. 2024 Jul 25;206(7):e0044223. doi: 10.1128/jb.00442-23. Epub 2024 Jun 4. J Bacteriol. 2024. PMID: 38832786 Free PMC article. Review.
-
The PilB-PilZ-FimX regulatory complex of the Type IV pilus from Xanthomonas citri.PLoS Pathog. 2021 Aug 16;17(8):e1009808. doi: 10.1371/journal.ppat.1009808. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34398935 Free PMC article.
-
Type IV pilus assembly in Pseudomonas aeruginosa over a broad range of cyclic di-GMP concentrations.J Bacteriol. 2012 Aug;194(16):4285-94. doi: 10.1128/JB.00803-12. Epub 2012 Jun 8. J Bacteriol. 2012. PMID: 22685276 Free PMC article.
-
Role of Cyclic Di-GMP and Exopolysaccharide in Type IV Pilus Dynamics.J Bacteriol. 2017 Mar 28;199(8):e00859-16. doi: 10.1128/JB.00859-16. Print 2017 Apr 15. J Bacteriol. 2017. PMID: 28167523 Free PMC article.
-
Building permits-control of type IV pilus assembly by PilB and its cofactors.J Bacteriol. 2024 Dec 19;206(12):e0035924. doi: 10.1128/jb.00359-24. Epub 2024 Nov 7. J Bacteriol. 2024. PMID: 39508682 Free PMC article. Review.
Cited by
-
Coordinated control of the type IV pili and c-di-GMP-dependent antifungal antibiotic production in Lysobacter by the response regulator PilR.Mol Plant Pathol. 2021 May;22(5):602-617. doi: 10.1111/mpp.13046. Epub 2021 Mar 11. Mol Plant Pathol. 2021. PMID: 33709522 Free PMC article.
-
Bacterial cell surface characterization by phage display coupled to high-throughput sequencing.Nat Commun. 2024 Aug 29;15(1):7502. doi: 10.1038/s41467-024-51912-7. Nat Commun. 2024. PMID: 39209859 Free PMC article.
-
Two antagonistic response regulators control Pseudomonas aeruginosa polarization during mechanotaxis.EMBO J. 2023 Apr 3;42(7):e112165. doi: 10.15252/embj.2022112165. Epub 2023 Feb 16. EMBO J. 2023. PMID: 36795017 Free PMC article.
-
Mechanotaxis directs Pseudomonas aeruginosa twitching motility.Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2101759118. doi: 10.1073/pnas.2101759118. Proc Natl Acad Sci U S A. 2021. PMID: 34301869 Free PMC article.
-
A bacterial sense of touch: T4P retraction motor as a means of surface sensing by Pseudomonas aeruginosa PA14.J Bacteriol. 2024 Jul 25;206(7):e0044223. doi: 10.1128/jb.00442-23. Epub 2024 Jun 4. J Bacteriol. 2024. PMID: 38832786 Free PMC article. Review.
References
-
- Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938 . - DOI - PubMed
-
- Craig L, Pique ME, Tainer JA. Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol. 2004;2(5):363–78. doi: 10.1038/nrmicro885 . - DOI - PubMed
-
- Chen I, Dubnau D. DNA uptake during bacterial transformation. Nat Rev Microbiol. 2004;2(3):241–9. doi: 10.1038/nrmicro844 . - DOI - PubMed
-
- Maier B, Potter L, So M, Long CD, Seifert HS, Sheetz MP. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A. 2002;99(25):16012–7. doi: 10.1073/pnas.242523299 . - DOI - PMC - PubMed
-
- Wolfgang M, van Putten JP, Hayes SF, Dorward D, Koomey M. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 2000;19(23):6408–18. doi: 10.1093/emboj/19.23.6408 . - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

