NEIL3 Repairs Telomere Damage during S Phase to Secure Chromosome Segregation at Mitosis
- PMID: 28854357
- PMCID: PMC5606162
- DOI: 10.1016/j.celrep.2017.08.020
NEIL3 Repairs Telomere Damage during S Phase to Secure Chromosome Segregation at Mitosis
Abstract
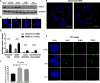
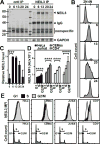
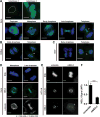
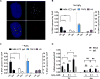
Oxidative damage to telomere DNA compromises telomere integrity. We recently reported that the DNA glycosylase NEIL3 preferentially repairs oxidative lesions in telomere sequences in vitro. Here, we show that loss of NEIL3 causes anaphase DNA bridging because of telomere dysfunction. NEIL3 expression increases during S phase and reaches maximal levels in late S/G2. NEIL3 co-localizes with TRF2 and associates with telomeres during S phase, and this association increases upon oxidative stress. Mechanistic studies reveal that NEIL3 binds to single-stranded DNA via its intrinsically disordered C terminus in a telomere-sequence-independent manner. Moreover, NEIL3 is recruited to telomeres through its interaction with TRF1, and this interaction enhances the enzymatic activity of purified NEIL3. Finally, we show that NEIL3 interacts with AP Endonuclease 1 (APE1) and the long-patch base excision repair proteins PCNA and FEN1. Taken together, we propose that NEIL3 protects genome stability through targeted repair of oxidative damage in telomeres during S/G2 phase.
Keywords: DNA repair; NEIL3 glycosylase; mitotic defects; telomere.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Chen Y, Yang Y, van Overbeek M, Donigian JR, Baciu P, de Lange T, Lei M. A shared docking motif in TRF1 and TRF2 used for differential recruitment of telomeric proteins. Science. 2008;319:1092–1096. - PubMed
-
- Chin K, de Solorzano CO, Knowles D, Jones A, Chou W, Rodriguez EG, Kuo WL, Ljung BM, Chew K, Myambo K, et al. In situ analyses of genome instability in breast cancer. Nat Genet. 2004;36:984–988. - PubMed
-
- de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

