Electroencephalography and delirium in the postoperative period
- PMID: 28854540
- PMCID: PMC6172974
- DOI: 10.1093/bja/aew475
Electroencephalography and delirium in the postoperative period
Abstract
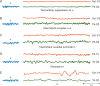
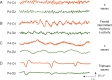
Delirium commonly manifests in the postoperative period as a clinical syndrome resulting from acute brain dysfunction or encephalopathy. Delirium is characterized by acute and often fluctuating changes in attention and cognition. Emergence delirium typically presents and resolves within minutes to hours after termination of general anaesthesia. Postoperative delirium hours to days after an invasive procedure can herald poor outcomes. Easily recognized when patients are hyperactive or agitated, delirium often evades diagnosis as it most frequently presents with hypoactivity and somnolence. EEG offers objective measurements to complement clinical assessment of this complex fluctuating disorder. Although EEG features of delirium in the postoperative period remain incompletely characterized, a shift of EEG power into low frequencies is a typical finding shared among encephalopathies that manifest with delirium. In aggregate, existing data suggest that serial or continuous EEG in the postoperative period facilitates monitoring of delirium development and severity and assists in detecting epileptic aetiologies. Future studies are needed to clarify the precise EEG features that can reliably predict or diagnose delirium in the postoperative period, and to provide mechanistic insights into this pathologically diverse neurological disorder.
Keywords: delirium; electroencephalography; encephalopathy.
© The Author 2017. Published by Oxford University Press on behalf of the British Journal of Anaesthesia. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Edn. American Psychiatric Association; Washington, DC: 2013.
-
- American Psychiatric Association . Practice Guideline for the Treatment of Patients with Delirium. American Psychiatric Association; Washington, DC: 2010.
-
- Administration on Aging . A profile of older Americans. Department of Health and Human Services; Washington, DC: 2000.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

