Aqua-soluble DDQ reduces the levels of Drp1 and Aβ and inhibits abnormal interactions between Aβ and Drp1 and protects Alzheimer's disease neurons from Aβ- and Drp1-induced mitochondrial and synaptic toxicities
- PMID: 28854701
- PMCID: PMC5886305
- DOI: 10.1093/hmg/ddx226
Aqua-soluble DDQ reduces the levels of Drp1 and Aβ and inhibits abnormal interactions between Aβ and Drp1 and protects Alzheimer's disease neurons from Aβ- and Drp1-induced mitochondrial and synaptic toxicities
Expression of concern in
-
Expression of Concern: Aqua-soluble DDQ reduces the levels of Drp1 and Aβ and inhibits abnormal interactions between Aβ and Drp1 and protects Alzheimer's disease neurons from Aβ- and Drp1-induced mitochondrial and synaptic toxicities.Hum Mol Genet. 2025 Feb 17;34(5):468. doi: 10.1093/hmg/ddae204. Hum Mol Genet. 2025. PMID: 39840519 Free PMC article. No abstract available.
Abstract
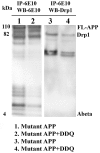
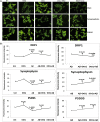
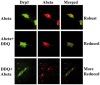
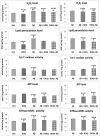
The purpose of our study was to develop a therapeutic target that can reduce Aβ and Drp1 levels, and also can inhibit abnormal interactions between Aβ and Drp1 in AD neurons. To achieve this objective, we designed various compounds and their 3-dimensional molecular structures were introduced into Aβ and Drp1 complex and identified their inhibitory properties against Aβ-Drp1 interaction. Among all, DDQ was selected for further investigation because of 1) its best docking score and 2) its binding capability at interacting sites of Drp1 and Aβ complex. We synthesized DDQ using retro-synthesis and analyzed its structure spectrally. Using biochemical, molecular biology, immunostaining and transmission electron microscopy (TEM) methods, we studied DDQ's beneficial effects in AD neurons. We measured the levels of Aβ and Drp1, Aβ and Drp1 interaction, mRNA and protein levels of mitochondrial dynamics, biogenesis and synaptic genes, mitochondrial function and cell viability and mitochondrial number in DDQ-treated and untreated AD neurons. Our qRT-PCR and immunoblotting analysis revealed that reduced levels of mitochondrial fission and increased fusion, biogenesis and synaptic genes in DDQ-treated AD neurons. Our immunoblotting and immunostaining analyses revealed that Aβ and Drp1 levels were reduced in DDQ-treated AD neurons. Interaction between Aβ and Drp1 is reduced in DDQ-treated AD neurons. Aβ42 levels were significantly reduced in DDQ-treated mutant APPSwe/Ind cells. Mitochondrial number is significantly reduced and mitochondrial length is significantly increased. Mitochondrial function and cell viability were maintained in AD neurons treated with DDQ. These observations indicate that DDQ reduces excessive mitochondrial fragmentation, enhances fusion, biogenesis and synaptic activity and reduces Aβ42 levels and protects AD neurons against Aβ-induced mitochondrial and synaptic toxicities.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures







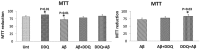
Similar articles
-
Protective effects of reduced dynamin-related protein 1 against amyloid beta-induced mitochondrial dysfunction and synaptic damage in Alzheimer's disease.Hum Mol Genet. 2016 Dec 1;25(23):5148-5166. doi: 10.1093/hmg/ddw330. Hum Mol Genet. 2016. PMID: 27677309 Free PMC article.
-
Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage.Hum Mol Genet. 2011 Jul 1;20(13):2495-509. doi: 10.1093/hmg/ddr139. Epub 2011 Mar 31. Hum Mol Genet. 2011. PMID: 21459773 Free PMC article.
-
Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: implications for mitochondrial dysfunction and neuronal damage.Hum Mol Genet. 2012 Jun 1;21(11):2538-47. doi: 10.1093/hmg/dds072. Epub 2012 Feb 24. Hum Mol Genet. 2012. PMID: 22367970 Free PMC article.
-
Multiple faces of dynamin-related protein 1 and its role in Alzheimer's disease pathogenesis.Biochim Biophys Acta. 2016 Apr;1862(4):814-828. doi: 10.1016/j.bbadis.2015.12.018. Epub 2015 Dec 17. Biochim Biophys Acta. 2016. PMID: 26708942 Free PMC article. Review.
-
S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration.Mitochondrion. 2010 Aug;10(5):573-8. doi: 10.1016/j.mito.2010.04.007. Epub 2010 May 4. Mitochondrion. 2010. PMID: 20447471 Free PMC article. Review.
Cited by
-
Regulation of mitochondrial dysfunction induced cell apoptosis is a potential therapeutic strategy for herbal medicine to treat neurodegenerative diseases.Front Pharmacol. 2022 Sep 22;13:937289. doi: 10.3389/fphar.2022.937289. eCollection 2022. Front Pharmacol. 2022. PMID: 36210852 Free PMC article. Review.
-
Reassessment of Pioglitazone for Alzheimer's Disease.Front Neurosci. 2021 Jun 16;15:666958. doi: 10.3389/fnins.2021.666958. eCollection 2021. Front Neurosci. 2021. PMID: 34220427 Free PMC article. Review.
-
Recent advancements in the understanding of the alterations in mitochondrial biogenesis in Alzheimer's disease.Mol Biol Rep. 2025 Jan 29;52(1):173. doi: 10.1007/s11033-025-10297-6. Mol Biol Rep. 2025. PMID: 39880979 Review.
-
Mitochondrial dysfunction and Alzheimer's disease: prospects for therapeutic intervention.BMB Rep. 2020 Jan;53(1):47-55. doi: 10.5483/BMBRep.2020.53.1.279. BMB Rep. 2020. PMID: 31818365 Free PMC article. Review.
-
Disturbances of mitochondrial dynamics in cultured neurons infected with human herpesvirus type 1 and type 2.J Neurovirol. 2019 Dec;25(6):765-782. doi: 10.1007/s13365-019-00762-x. Epub 2019 Jun 3. J Neurovirol. 2019. PMID: 31161588 Free PMC article.
References
-
- World Alzheimer Report 2015: The Global Impact of Dementia.
-
- LaFerla F.M., Green K.N., Oddo S. (2007) Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci., 8, 499–509. - PubMed
-
- Selkoe D.J. (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev., 81, 741–766. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

