Addition of nucleoside analogues to peg-IFNα-2a enhances virological response in chronic hepatitis B patients without early response to peg-IFNα-2a: a randomized controlled trial
- PMID: 28854883
- PMCID: PMC5577782
- DOI: 10.1186/s12876-017-0657-y
Addition of nucleoside analogues to peg-IFNα-2a enhances virological response in chronic hepatitis B patients without early response to peg-IFNα-2a: a randomized controlled trial
Abstract
Background: Current treatments for chronic hepatitis B (CHB) include pegylated interferon alpha (PEG-IFN-α) which is an immune modulator, and nucleos(t)ide analogs (NAs) which directly inhibit HBV DNA polymerase. With the limited efficacy of PEG-IFN-α and prolonged treatment periods associated with NAs, there is an urgent need for novel therapeutic strategies, especially for patients with a poor early response to anti-viral therapy.
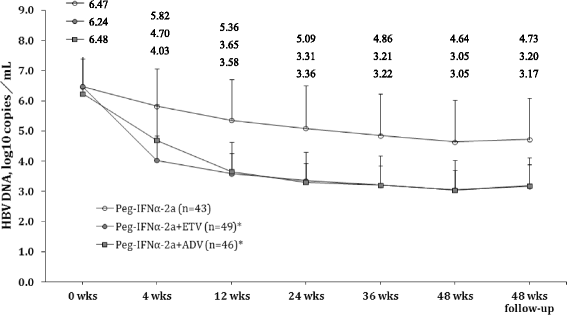
Methods: In this study, 178 patients with chronic hepatitis B (n = 131) and compensated (n = 47) HBV-induced cirrhosis were enrolled, 120 patients with HBeAg (+). All the patients were treated for 12 weeks with PEG-IFN-α. Among them, a total of 138 patients with a poor virological response after 12 weeks were treated for an additional 48 weeks with Peg-IFNα-2a (control) (n = 43), with Peg-IFNα-2a + entecavir (ETV) (n = 49), or Peg-IFNα-2a + adefovir dipivoxil (ADV) (n = 46), and were followed for 48 weeks after therapy. Early virological response was defined as undetectable HBV DNA after anti-viral therapy for 12 weeks. Sustained virological response (SVR) was defined as no change in therapeutic effectiveness after 6 months follow-up, and no recurrence.Therapeutic efficacy was determined by evaluating HBV DNA levels, serum and liver HBsAg levels, liver function tests and liver histology.
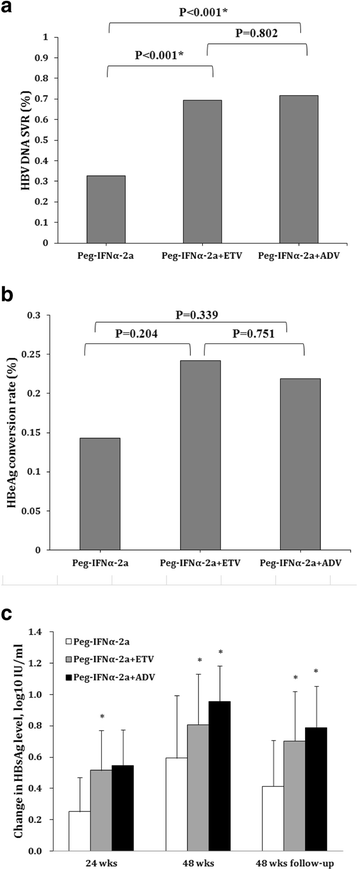
Results: Patients in the Peg-IFNα-2a + ETV and Peg-IFNα-2a + ADV groups showed a significantly greater decrease in HBV DNA levels over time, and a significantly higher SVR compared to patients receiving Peg-INFα-2a monotherapy (both P values <0.05). Although patients receiving combination therapy had a significantly higher change in serum HBsAg levels compared to the monotherapy group, there was no significant difference in liver HBsAg levels between the three treatment groups.
Conclusion: This study demonstrated that in patients with a poor virological response after 12 weeks of treatment with Peg-IFNα-2a alone, addition of ADV or ETV significantly reduced HBV DNA levels, serum HBsAg levels, and increased SVR. Individualization of anti-viral therapy would ensure that only patients who do not respond to Peg-IFNα-2a would receive combination therapy. Our data have important implications for the treatment of CHB patients who fail to show an early response to Peg-IFNα-2a monotherapy.
Trial registration: This trial was retrospectively registered on 2012 May 24 at the China Clinical Trials Registry (ChiCTR-OCC-12002196).
Keywords: Chronic hepatitis B; Combination therapy; Interferon; Nucleoside analog; Virological response.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by a central independent ethics committee of China-Japan Union Hospital of Jilin University. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
Writing assistance was sponsored by Shanghai Roche Pharmaceuticals Ltd.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

