Spatial and temporal epithelial ovarian cancer cell heterogeneity impacts Maraba virus oncolytic potential
- PMID: 28854921
- PMCID: PMC5577660
- DOI: 10.1186/s12885-017-3600-2
Spatial and temporal epithelial ovarian cancer cell heterogeneity impacts Maraba virus oncolytic potential
Abstract
Background: Epithelial ovarian cancer exhibits extensive interpatient and intratumoral heterogeneity, which can hinder successful treatment strategies. Herein, we investigated the efficacy of an emerging oncolytic, Maraba virus (MRBV), in an in vitro model of ovarian tumour heterogeneity.
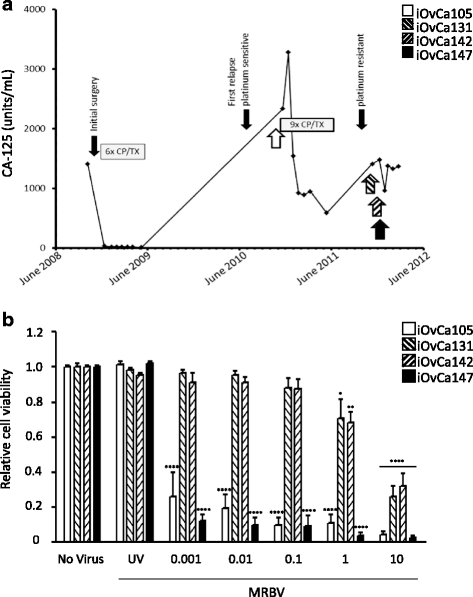
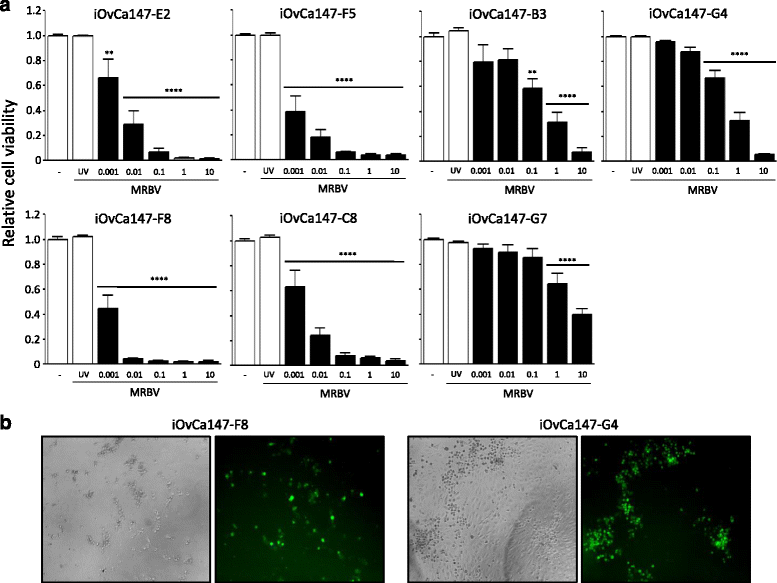
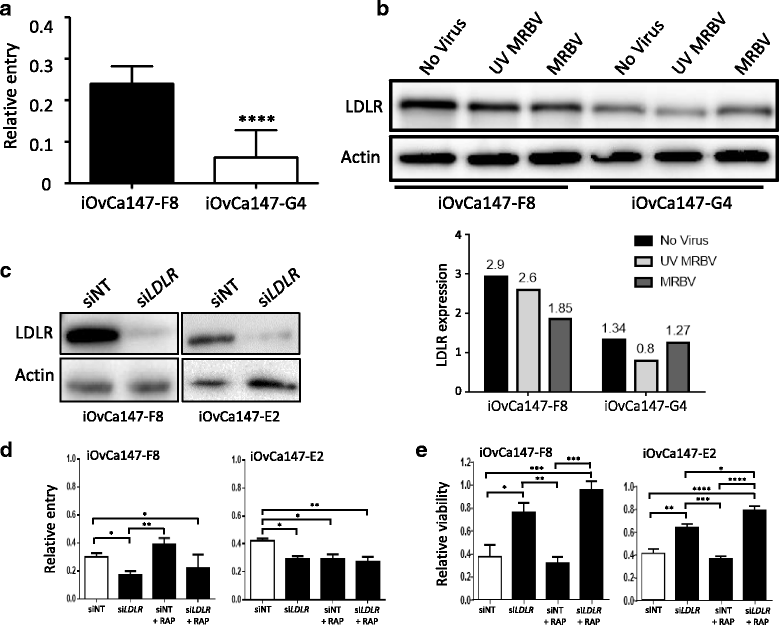
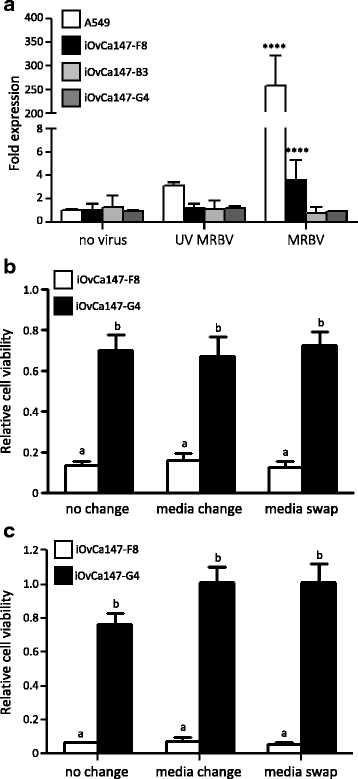
Methods: Four ovarian high-grade serous cancer (HGSC) cell lines were isolated and established from a single patient at four points during disease progression. Limiting-dilution subcloning generated seven additional subclone lines to assess intratumoral heterogeneity. MRBV entry and oncolytic efficacy were assessed among all 11 cell lines. Low-density receptor (LDLR) expression, conditioned media treatments and co-cultures were performed to determine factors impacting MRBV oncolysis.
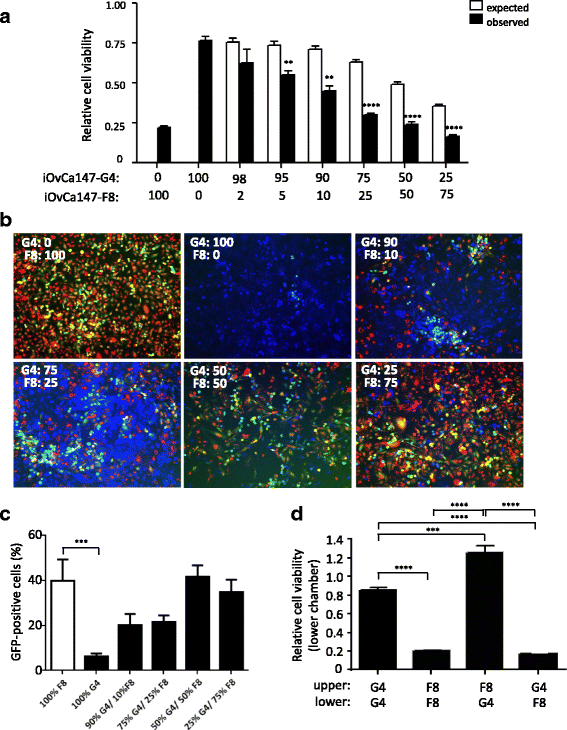
Results: Temporal and intratumoral heterogeneity identified two subpopulations of cells: one that was highly sensitive to MRBV, and another set which exhibited 1000-fold reduced susceptibility to MRBV-mediated oncolysis. We explored both intracellular and extracellular mechanisms influencing sensitivity to MRBV and identified that LDLR can partially mediate MRBV infection. LDLR expression, however, was not the singular determinant of sensitivity to MRBV among the HGSC cell lines and subclones. We verified that there were no apparent extracellular factors, such as type I interferon responses, contributing to MRBV resistance. However, direct cell-cell contact by co-culture of MRBV-resistant subclones with sensitive cells restored virus infection and oncolytic killing of mixed population.
Conclusions: Our data is the first to demonstrate differential efficacy of an oncolytic virus in the context of both spatial and temporal heterogeneity of HGSC cells and to evaluate whether it will constitute a barrier to effective viral oncolytic therapy.
Keywords: Ascites; High-grade serous ovarian cancer; Maraba virus; Oncolytic virus; Resistance; Tumour heterogeneity.
Conflict of interest statement
Ethics approval and consent to participate
All patient-derived cells were used in accordance with The University of Western Ontario Human Research Ethics Board approved protocol (UWO HSREB 12668E). Written informed consent was obtained from all individuals to use their cells in research.
Consent for publication
Not applicable.
Competing interests
JC Bell and D Stojdl have a patent on Maraba as an Oncolytic Virus licensed to Turnstone Biologics.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Myxoma virus-mediated oncolysis of ascites-derived human ovarian cancer cells and spheroids is impacted by differential AKT activity.Gynecol Oncol. 2012 May;125(2):441-50. doi: 10.1016/j.ygyno.2012.01.048. Epub 2012 Feb 1. Gynecol Oncol. 2012. PMID: 22306204 Free PMC article.
-
A phase 1b study of intraperitoneal oncolytic viral immunotherapy in platinum-resistant or refractory ovarian cancer.Gynecol Oncol. 2021 Dec;163(3):481-489. doi: 10.1016/j.ygyno.2021.10.069. Epub 2021 Oct 20. Gynecol Oncol. 2021. PMID: 34686353 Clinical Trial.
-
Treatment of chemotherapy resistant ovarian cancer with a MDR1 targeted oncolytic adenovirus.Gynecol Oncol. 2011 Oct;123(1):138-46. doi: 10.1016/j.ygyno.2011.06.007. Epub 2011 Jul 13. Gynecol Oncol. 2011. PMID: 21741695
-
Measles to the Rescue: A Review of Oncolytic Measles Virus.Viruses. 2016 Oct 22;8(10):294. doi: 10.3390/v8100294. Viruses. 2016. PMID: 27782084 Free PMC article. Review.
-
Sindbis Virus Vaccine Platform: A Promising Oncolytic Virus-Mediated Approach for Ovarian Cancer Treatment.Int J Mol Sci. 2024 Mar 2;25(5):2925. doi: 10.3390/ijms25052925. Int J Mol Sci. 2024. PMID: 38474178 Free PMC article. Review.
Cited by
-
Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy.Nat Protoc. 2024 Sep;19(9):2540-2570. doi: 10.1038/s41596-024-00985-1. Epub 2024 May 20. Nat Protoc. 2024. PMID: 38769145 Review.
-
Oncolytic Viruses in Ovarian Cancer: Where Do We Stand? A Narrative Review.Pathogens. 2025 Feb 3;14(2):140. doi: 10.3390/pathogens14020140. Pathogens. 2025. PMID: 40005517 Free PMC article. Review.
-
Netrin signaling mediates survival of dormant epithelial ovarian cancer cells.Elife. 2024 Jul 18;12:RP91766. doi: 10.7554/eLife.91766. Elife. 2024. PMID: 39023520 Free PMC article.
-
HIV-Infected Macrophages Are Infected and Killed by the Interferon-Sensitive Rhabdovirus MG1.J Virol. 2021 Apr 12;95(9):e01953-20. doi: 10.1128/JVI.01953-20. Print 2021 Apr 12. J Virol. 2021. PMID: 33568507 Free PMC article.
-
Enhancing oncolytic virotherapy by extracellular vesicle mediated microRNA reprograming of the tumour microenvironment.Front Immunol. 2024 Dec 23;15:1500570. doi: 10.3389/fimmu.2024.1500570. eCollection 2024. Front Immunol. 2024. PMID: 39763667 Free PMC article.
References
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2011. Bethesda: National Cancer Institute, National Institutes of Health; 2014.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

