Exosomes isolated from cancer patients' sera transfer malignant traits and confer the same phenotype of primary tumors to oncosuppressor-mutated cells
- PMID: 28854931
- PMCID: PMC5577828
- DOI: 10.1186/s13046-017-0587-0
Exosomes isolated from cancer patients' sera transfer malignant traits and confer the same phenotype of primary tumors to oncosuppressor-mutated cells
Abstract
Background: Horizontal transfer of malignant traits from the primary tumor to distant organs, through blood circulating factors, has recently become a thoroughly studied metastatic pathway to explain cancer dissemination. Recently, we reported that oncosuppressor gene-mutated human cells undergo malignant transformation when exposed to cancer patients' sera. We also observed that oncosuppressor mutated cells would show an increased uptake of cancer-derived exosomes and we suggested that oncosuppressor genes might protect the integrity of the cell genome by blocking integration of cancer-derived exosomes. In the present study, we tested the hypothesis that cancer patients' sera-derived exosomes might be responsible for the malignant transformation of target cells and that oncosuppressor mutation would promote their increased uptake. We also sought to unveil the mechanisms behind the hypothesized phenomena.
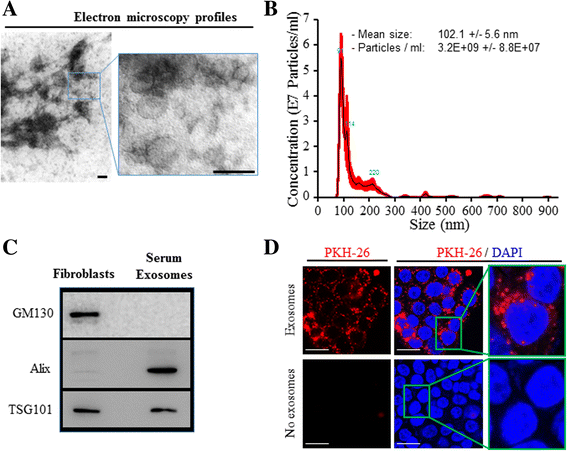
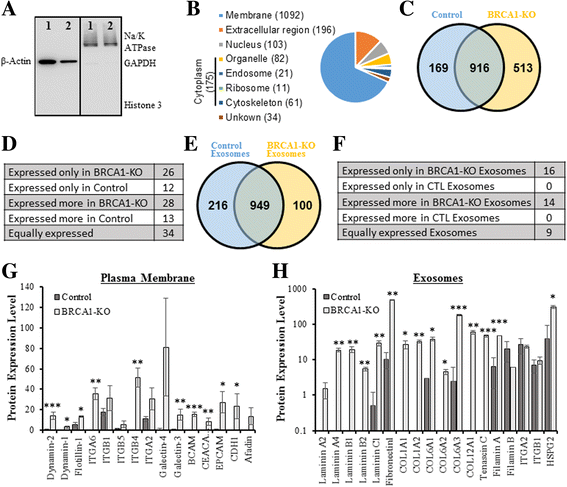
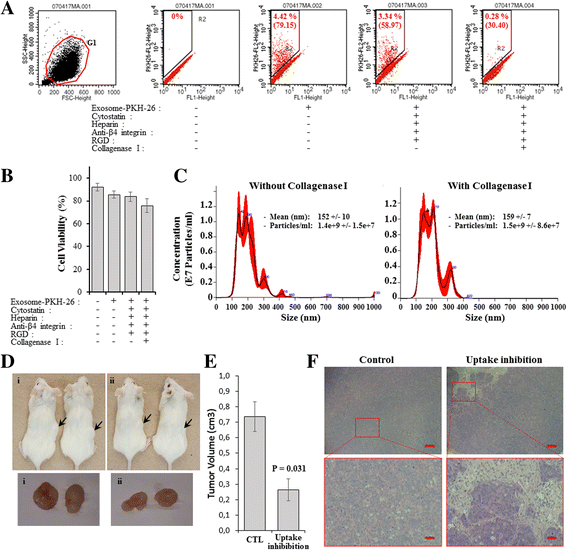
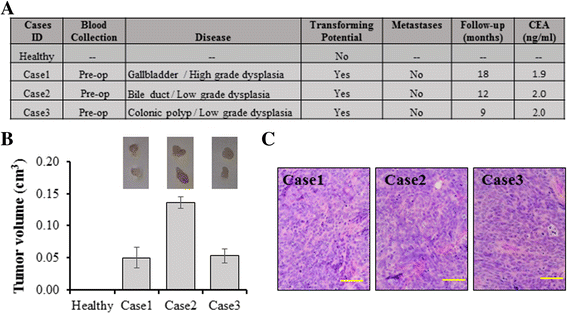
Methods: We used human BRCA1 knockout (BRCA1-KO) fibroblasts as target cells. Cells were treated in vitro with cancer patients' sera or cancer patients' sera-derived exosomes. Treated cells were injected into NOD-SCID mice. Immunohistochemical analyses were performed to determine the differentiation state of the xenotransplants. Mass spectrometry analyses of proteins from cancer exosomes and the BRCA1-KO fibroblasts' membrane were performed to investigate possible de novo expression of molecules involved in vesicles uptake. Blocking of the identified molecules in vitro was performed and in vivo experiments were conducted to confirm the role of these molecules in the malignant transformation carried out by cancer-derived exosomes.
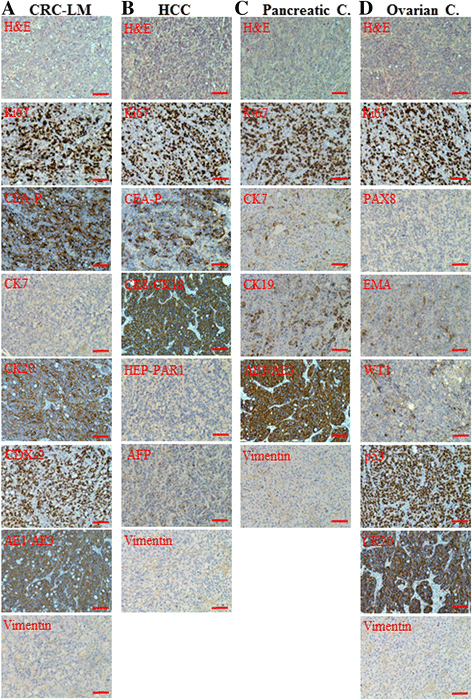
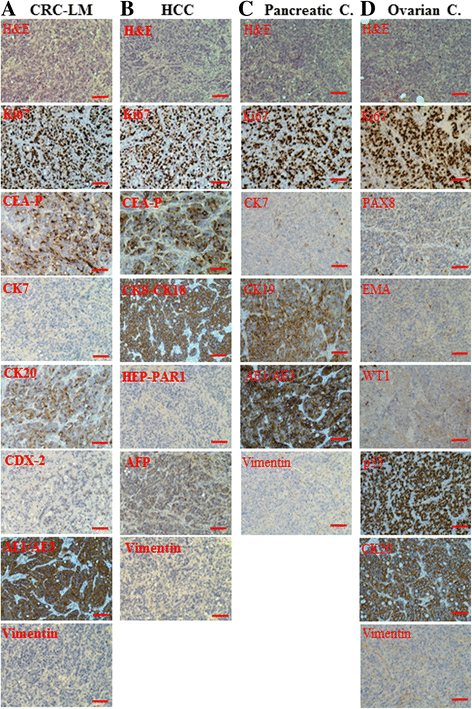
Results: Cells treated with exosomes isolated from cancer patients' sera underwent malignant transformation and formed tumors when transplanted into immunodeficient mice. Histological analyses showed that the tumors were carcinomas that differentiated into the same lineage of the primary tumors of blood donors. Oncosuppressor mutation promoted the de novo expression, on the plasma membrane of target cells, of receptors, responsible for the increased uptake of cancer-derived exosomes. The selective blocking of these receptors inhibited the horizontal transfer of malignant traits.
Conclusion: These findings strengthen the hypothesis that oncogenic factors transferred via circulating cancer exosomes, induce malignant transformation of target cells even at distance. Oncosuppressor genes might protect the integrity of the cell genome by inhibiting the uptake of cancer-derived exosomes.
Keywords: Cancer patients’ serum; Exosomes; Genometastasis; Horizontal transfer; Malignant transformation; Phenotypical differentiation; Tumor suppressor genes.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

