Nuclear envelopathies: a complex LINC between nuclear envelope and pathology
- PMID: 28854936
- PMCID: PMC5577761
- DOI: 10.1186/s13023-017-0698-x
Nuclear envelopathies: a complex LINC between nuclear envelope and pathology
Abstract
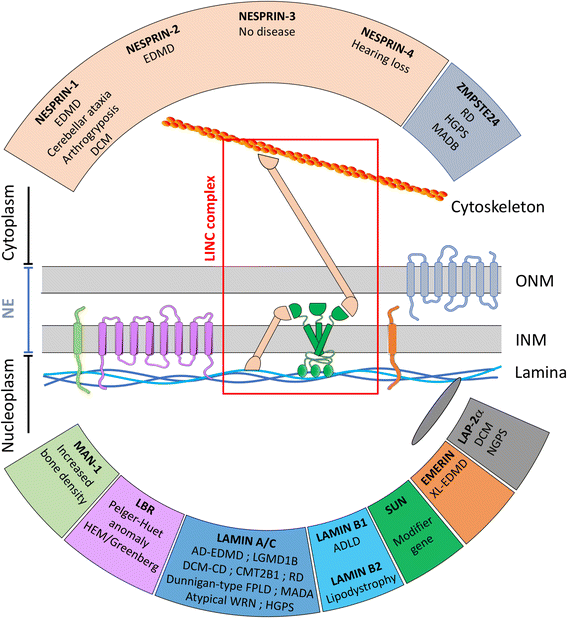
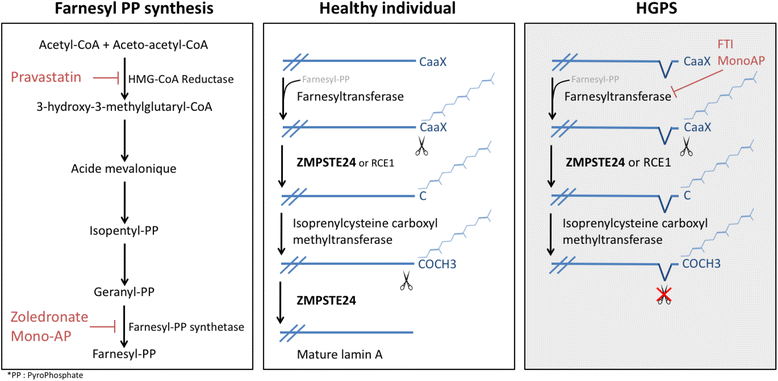
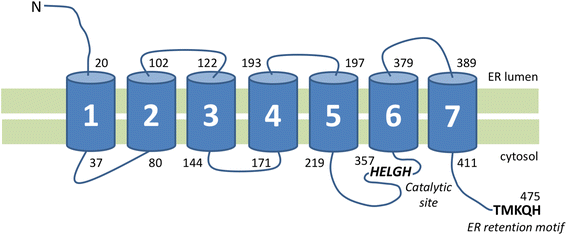
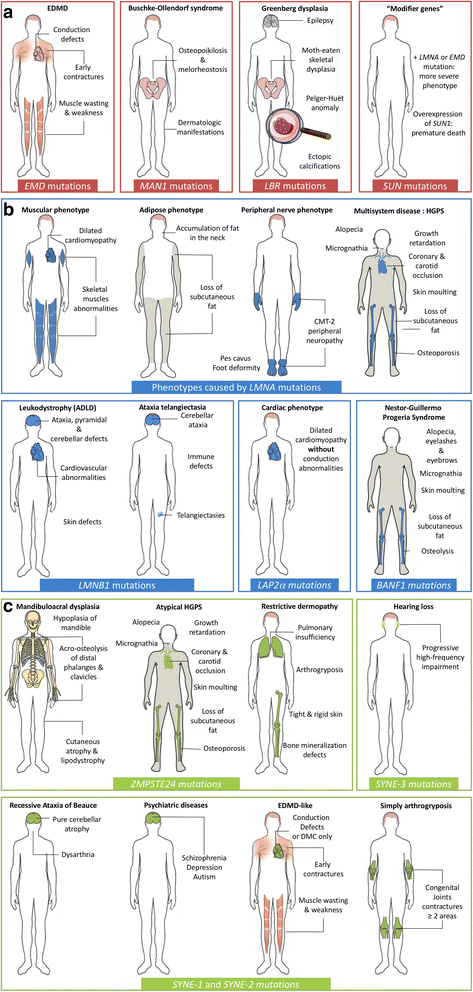
Since the identification of the first disease causing mutation in the gene coding for emerin, a transmembrane protein of the inner nuclear membrane, hundreds of mutations and variants have been found in genes encoding for nuclear envelope components. These proteins can be part of the inner nuclear membrane (INM), such as emerin or SUN proteins, outer nuclear membrane (ONM), such as Nesprins, or the nuclear lamina, such as lamins A and C. However, they physically interact with each other to insure the nuclear envelope integrity and mediate the interactions of the nuclear envelope with both the genome, on the inner side, and the cytoskeleton, on the outer side. The core of this complex, called LINC (LInker of Nucleoskeleton to Cytoskeleton) is composed of KASH and SUN homology domain proteins. SUN proteins are INM proteins which interact with lamins by their N-terminal domain and with the KASH domain of nesprins located in the ONM by their C-terminal domain.Although most of these proteins are ubiquitously expressed, their mutations have been associated with a large number of clinically unrelated pathologies affecting specific tissues. Moreover, variants in SUN proteins have been found to modulate the severity of diseases induced by mutations in other LINC components or interactors. For these reasons, the diagnosis and the identification of the molecular explanation of "nuclear envelopathies" is currently challenging.The aim of this review is to summarize the human diseases caused by mutations in genes coding for INM proteins, nuclear lamina, and ONM proteins, and to discuss their potential physiopathological mechanisms that could explain the large spectrum of observed symptoms.
Keywords: EMD; LAP2; LINC complex; LMNA; Lamins a/C; Nesprins; Nuclear envelope; Sun; ZMPSTE24.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of the paper.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

