Innovative dressing and securement of tunneled central venous access devices in pediatrics: a pilot randomized controlled trial
- PMID: 28854967
- PMCID: PMC5577834
- DOI: 10.1186/s12885-017-3606-9
Innovative dressing and securement of tunneled central venous access devices in pediatrics: a pilot randomized controlled trial
Abstract
Background: Central venous access device (CVAD) associated complications are a preventable source of patient harm, frequently resulting in morbidity and delays to vital treatment. Dressing and securement products are used to prevent infectious and mechanical complications, however current complication rates suggest customary practices are inadequate. The aim of this study was to evaluate the feasibility of launching a full-scale randomized controlled efficacy trial of innovative dressing and securement products for pediatric tunneled CVAD to prevent complication and failure.
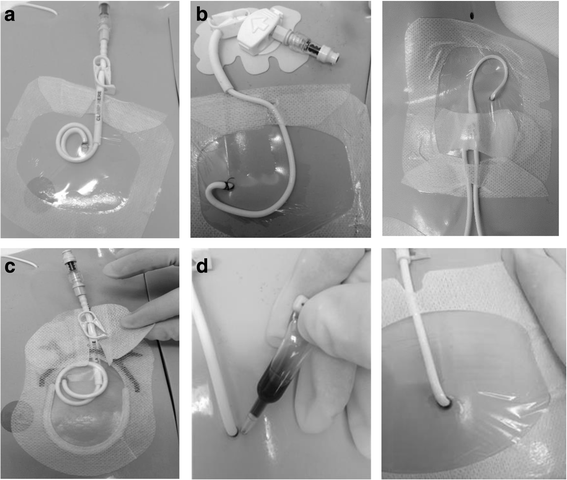
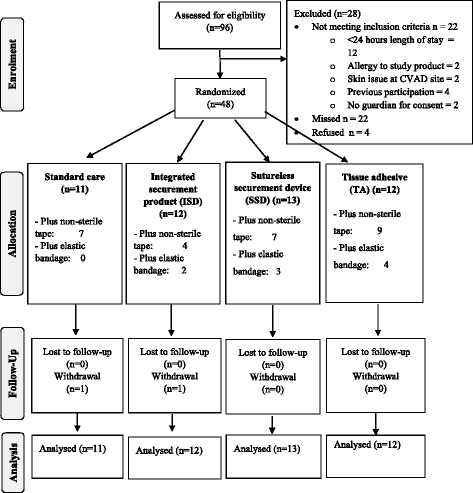
Methods: An external, pilot, four-group randomized controlled trial of standard care (bordered polyurethane dressing and suture), in comparison to integrated securement-dressing, suture-less securement device, and tissue adhesive was undertaken across two large, tertiary referral pediatric hospitals in Australia. Forty-eight pediatric participants with newly inserted tunneled CVADs were consecutively recruited. The primary outcome of study feasibility was established by elements of eligibility, recruitment, attrition, protocol adherence, missing data, parent and healthcare staff satisfaction and acceptability, and effect size estimates for CVAD failure (cessation of function prior to completion of treatment) and complication (associated bloodstream infection, thrombosis, breakage, dislodgement or occlusion). Dressing integrity, product costs and site complications were also examined.
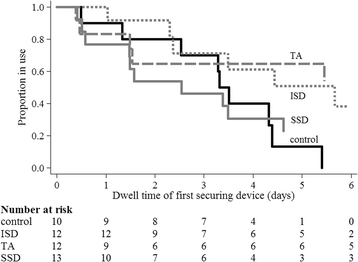
Results: Protocol feasibility was established. CVAD failure was: 17% (2/12) integrated securement-dressing; 8% (1/13) suture-less securement device; 0% tissue adhesive (0/12); and, 0% standard care (0/11). CVAD complications were: 15% (2/13) suture-less securement device (CVAD associated bloodstream infection, and occlusion and partial dislodgement); 8% (1/12) integrated securement-dressing (partial dislodgement); 0% tissue adhesive (0/12); and, 0% standard care (0/11). One CVAD-associated bloodstream infection occurred, within the suture-less securement device group. Overall satisfaction was highest in the integrated securement-dressing (mean 8.5/10; standard deviation 1.2). Improved dressing integrity was evident in the intervention arms, with the integrated securement-dressing associated with prolonged time to first dressing change (mean days 3.5).
Conclusions: Improving the security and dressing integrity of tunneled CVADs is likely to improve outcomes for pediatric patients. Further research is necessary to identify novel, effective CVAD securement to reduce complications, and provide reliable vascular access for children.
Trial registration: ACTRN12614000280606 ; prospectively registered on 17/03/2014.
Keywords: Central venous catheter; Dressing; Evidence-based care; Pediatrics; Randomized controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval for the trial was gained from the Children’s Health Services Queensland (HREC/13/QRCH/181) and Griffith University (NRS/10/14/HREC) Human Research Ethics Committees (HREC). The trial was also registered with the Australian and New Zealand Clinical Trial Registry (ACTRN12614000280606). Informed consent from parents, legal guardians and children (youth assent, where appropriate) was gained prior to enrolment.
Consent for publication
Not applicable.
Competing interests
Griffith University has received research grants to support research projects undertaken by AJU, CRM, and TK from product manufacturers (3M, Adhezion, Angiodynamics, Becton Dickinson; BBraun Carefusion; Centurion Medical Products; Entrotech, Teleflex). MC has received an unrestricted educational grant from Baxter.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Chopra V, Flanders SA, Saint S, Woller SC, O'Grady NP, Safdar N, Trerotola SO, Saran R, Moureau N, Wiseman S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1–S40. doi: 10.7326/M15-0744. - DOI - PubMed
-
- Robinson JL, Casey LM, Huynh HQ, Spady DW. Prospective cohort study of the outcome of and risk factors for intravascular catheter-related bloodstream infections in children with intestinal failure. J Parenter Enteral Nutr. 2013;35(5):625–30. - PubMed
-
- Fratino G, Molinari AC, Parodi S, Longo S, Saracco P, Castagnola E, Haupt R. Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Annals of oncology : Official J Eur Soc Med Oncol / ESMO. 2005;16(4):648–654. doi: 10.1093/annonc/mdi111. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

