The neoepitope landscape in pediatric cancers
- PMID: 28854978
- PMCID: PMC5577668
- DOI: 10.1186/s13073-017-0468-3
The neoepitope landscape in pediatric cancers
Abstract
Background: Neoepitopes derived from tumor-specific somatic mutations are promising targets for immunotherapy in childhood cancers. However, the potential for such therapies in targeting these epitopes remains uncertain due to a lack of knowledge of the neoepitope landscape in childhood cancer. Studies to date have focused primarily on missense mutations without exploring gene fusions, which are a major class of oncogenic drivers in pediatric cancer.
Methods: We developed an analytical workflow for identification of putative neoepitopes based on somatic missense mutations and gene fusions using whole-genome sequencing data. Transcriptome sequencing data were incorporated to interrogate the expression status of the neoepitopes.
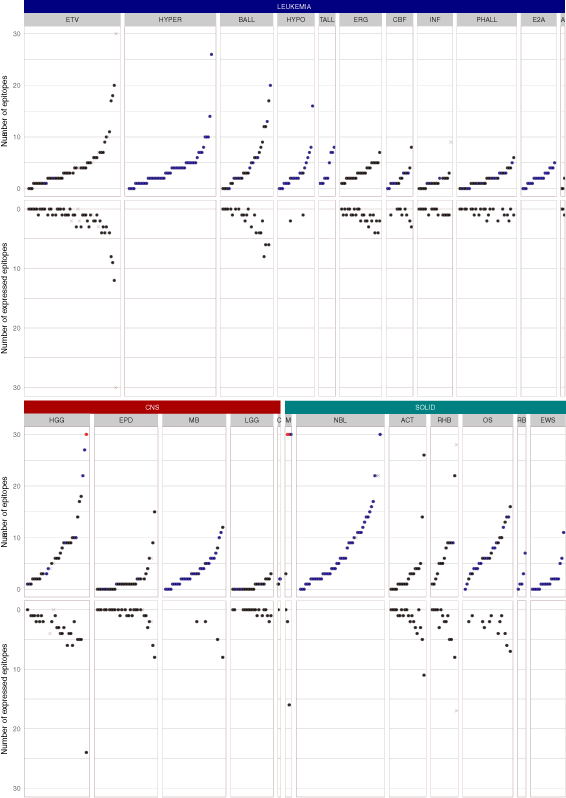
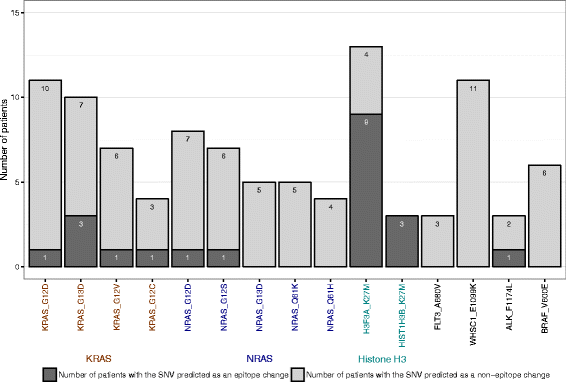
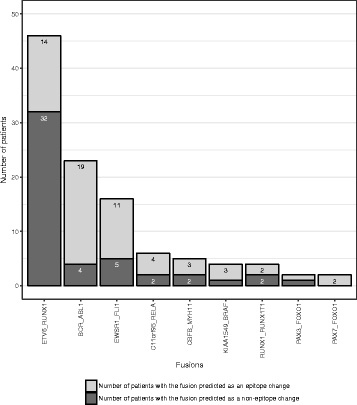
Results: We present the neoepitope landscape of somatic alterations including missense mutations and oncogenic gene fusions identified in 540 childhood cancer genomes and transcriptomes representing 23 cancer subtypes. We found that 88% of leukemias, 78% of central nervous system tumors, and 90% of solid tumors had at least one predicted neoepitope. Mutation hotspots in KRAS and histone H3 genes encode potential epitopes in multiple patients. Additionally, the ETV6-RUNX1 fusion was found to encode putative neoepitopes in a high proportion (69.6%) of the pediatric leukemia harboring this fusion.
Conclusions: Our study presents a comprehensive repertoire of potential neoepitopes in childhood cancers, and will facilitate the development of immunotherapeutic approaches designed to exploit them. The source code of the workflow is available at GitHub ( https://github.com/zhanglabstjude/neoepitope ).
Keywords: Epitopes; Gene fusions; Immunotherapy; Pediatric cancer.
Conflict of interest statement
Ethics approval and consent to participate
The use of human tissues for sequencing was approved by the institutional review board of St Jude Children’s Research Hospital in accordance with the principles of the Declaration of Helsinki. Written informed consent was provided by a parent or guardian of each child or by a patient who was 18 years of age or older.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Comment in
-
Relationship between sarcopenia and household status in community-dwelling older women.Geriatr Gerontol Int. 2017 Jan;17(1):179-180. doi: 10.1111/ggi.12837. Geriatr Gerontol Int. 2017. PMID: 28112494 No abstract available.
-
Clinical implications of neoepitope landscapes for adult and pediatric cancers.Genome Med. 2017 Aug 31;9(1):77. doi: 10.1186/s13073-017-0470-9. Genome Med. 2017. PMID: 28854952 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

