Reduced generation of lung tissue-resident memory T cells during infancy
- PMID: 28855242
- PMCID: PMC5626403
- DOI: 10.1084/jem.20170521
Reduced generation of lung tissue-resident memory T cells during infancy
Abstract
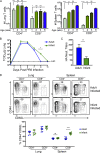
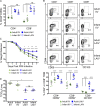
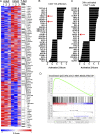
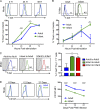
Infants suffer disproportionately from respiratory infections and generate reduced vaccine responses compared with adults, although the underlying mechanisms remain unclear. In adult mice, lung-localized, tissue-resident memory T cells (TRMs) mediate optimal protection to respiratory pathogens, and we hypothesized that reduced protection in infancy could be due to impaired establishment of lung TRM. Using an infant mouse model, we demonstrate generation of lung-homing, virus-specific T effectors after influenza infection or live-attenuated vaccination, similar to adults. However, infection during infancy generated markedly fewer lung TRMs, and heterosubtypic protection was reduced compared with adults. Impaired TRM establishment was infant-T cell intrinsic, and infant effectors displayed distinct transcriptional profiles enriched for T-bet-regulated genes. Notably, mouse and human infant T cells exhibited increased T-bet expression after activation, and reduction of T-bet levels in infant mice enhanced lung TRM establishment. Our findings reveal that infant T cells are intrinsically programmed for short-term responses, and targeting key regulators could promote long-term, tissue-targeted protection at this critical life stage.
© 2017 Zens et al.
Figures





References
-
- Banerjee A., Gordon S.M., Intlekofer A.M., Paley M.A., Mooney E.C., Lindsten T., Wherry E.J., and Reiner S.L.. 2010. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185:4988–4992. 10.4049/jimmunol.1002042 - DOI - PMC - PubMed
-
- Belshe R.B., Edwards K.M., Vesikari T., Black S.V., Walker R.E., Hultquist M., Kemble G., and Connor E.M.. CAIV-T Comparative Efficacy Study Group . 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 356:685–696. 10.1056/NEJMoa065368 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

