CDK8/19 Mediator kinases potentiate induction of transcription by NFκB
- PMID: 28855340
- PMCID: PMC5617299
- DOI: 10.1073/pnas.1710467114
CDK8/19 Mediator kinases potentiate induction of transcription by NFκB
Abstract
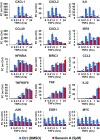
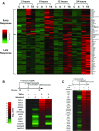
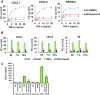
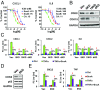
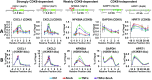
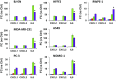
The nuclear factor-κB (NFκB) family of transcription factors has been implicated in inflammatory disorders, viral infections, and cancer. Most of the drugs that inhibit NFκB show significant side effects, possibly due to sustained NFκB suppression. Drugs affecting induced, but not basal, NFκB activity may have the potential to provide therapeutic benefit without associated toxicity. NFκB activation by stress-inducible cell cycle inhibitor p21 was shown to be mediated by a p21-stimulated transcription-regulating kinase CDK8. CDK8 and its paralog CDK19, associated with the transcriptional Mediator complex, act as coregulators of several transcription factors implicated in cancer; CDK8/19 inhibitors are entering clinical development. Here we show that CDK8/19 inhibition by different small-molecule kinase inhibitors or shRNAs suppresses the elongation of NFκB-induced transcription when such transcription is activated by p21-independent canonical inducers, such as TNFα. On NFκB activation, CDK8/19 are corecruited with NFκB to the promoters of the responsive genes. Inhibition of CDK8/19 kinase activity suppresses the RNA polymerase II C-terminal domain phosphorylation required for transcriptional elongation, in a gene-specific manner. Genes coregulated by CDK8/19 and NFκB include IL8, CXCL1, and CXCL2, which encode tumor-promoting proinflammatory cytokines. Although it suppressed newly induced NFκB-driven transcription, CDK8/19 inhibition in most cases had no effect on the basal expression of NFκB-regulated genes or promoters; the same selective regulation of newly induced transcription was observed with other transcription signals potentiated by CDK8/19. This selective role of CDK8/19 identifies these kinases as mediators of transcriptional reprogramming, a key aspect of development and differentiation as well as pathological processes.
Keywords: CDK19; CDK8; NFκB; RNA polymerase II; regulation of transcription.
Conflict of interest statement
Conflict of interest statement: M.C. and E.V.B. are consultants, D.C.P. is an employee, and I.B.R. is the founder and president of Senex Biotechnology, Inc.
Figures




References
-
- Poole JC, Thain A, Perkins ND, Roninson IB. Induction of transcription by p21Waf1/Cip1/Sdi1: Role of NFkappaB and effect of non-steroidal anti-inflammatory drugs. Cell Cycle. 2004;3:931–940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

