Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis
- PMID: 28855398
- PMCID: PMC5606244
- DOI: 10.1126/scitranslmed.aai8710
Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis
Abstract
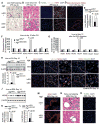
The regenerative capacity of lung and liver is sometimes impaired by chronic or overwhelming injury. Orthotopic transplantation of parenchymal stem cells to damaged organs might reinstate their self-repair ability. However, parenchymal cell engraftment is frequently hampered by the microenvironment in diseased recipient organs. We show that targeting both the vascular niche and perivascular fibroblasts establishes "hospitable soil" to foster the incorporation of "seed," in this case, the engraftment of parenchymal cells in injured organs. Specifically, ectopic induction of endothelial cell (EC)-expressed paracrine/angiocrine hepatocyte growth factor (HGF) and inhibition of perivascular NOX4 [NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase 4] synergistically enabled reconstitution of mouse and human parenchymal cells in damaged organs. Reciprocally, genetic knockout of Hgf in mouse ECs (HgfiΔEC/iΔEC) aberrantly up-regulated perivascular NOX4 during liver and lung regeneration. Dysregulated HGF and NOX4 pathways subverted the function of vascular and perivascular cells from an epithelially inductive niche to a microenvironment that inhibited parenchymal reconstitution. Perivascular NOX4 induction in HgfiΔEC/iΔEC mice recapitulated the phenotype of human and mouse liver and lung fibrosis. Consequently, EC-directed HGF and NOX4 inhibitor GKT137831 stimulated regenerative integration of mouse and human parenchymal cells in chronically injured lung and liver. Our data suggest that targeting dysfunctional perivascular and vascular cells in diseased organs can bypass fibrosis and enable reparative cell engraftment to reinstate lung and liver regeneration.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures







References
-
- Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, Terhardt D, Vogel MJ, Cao L, Korn C, Bartels S, Thomas M, Augustin HG. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. published online EpubJan 24. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

