Photoelectrochemical oxidation of organic substrates in organic media
- PMID: 28855502
- PMCID: PMC5577226
- DOI: 10.1038/s41467-017-00420-y
Photoelectrochemical oxidation of organic substrates in organic media
Abstract
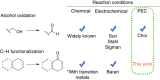
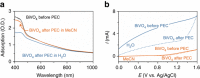
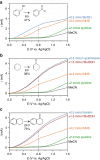
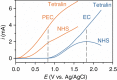
There is a global effort to convert sunlight into fuels by photoelectrochemically splitting water to form hydrogen fuels, but the dioxygen byproduct bears little economic value. This raises the important question of whether higher value commodities can be produced instead of dioxygen. We report here photoelectrochemistry at a BiVO4 photoanode involving the oxidation of substrates in organic media. The use of MeCN instead of water enables a broader set of chemical transformations to be performed (e.g., alcohol oxidation and C-H activation/oxidation), while suppressing photocorrosion of BiVO4 that otherwise occurs readily in water, and sunlight reduces the electrical energy required to drive organic transformations by 60%. These collective results demonstrate the utility of using photoelectrochemical cells to mediate organic transformations that otherwise require expensive and toxic reagents or catalysts.Photoelectrochemical water splitting is a promising method for H2 fuel production, but the O2 by-product generated has little economic value. Here, Berlinguette and colleagues demonstrate that BiVO4 photoanodes immersed in organic media can instead perform valuable alcohol oxidation and C-H functionalization reactions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

