Developing a molecular picture of soil organic matter-mineral interactions by quantifying organo-mineral binding
- PMID: 28855506
- PMCID: PMC5577185
- DOI: 10.1038/s41467-017-00407-9
Developing a molecular picture of soil organic matter-mineral interactions by quantifying organo-mineral binding
Erratum in
-
Author Correction: Developing a molecular picture of soil organic matter-mineral interactions by quantifying organo-mineral binding.Nat Commun. 2017 Dec 5;8(1):2017. doi: 10.1038/s41467-017-02125-8. Nat Commun. 2017. PMID: 29208904 Free PMC article.
Abstract
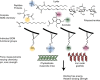
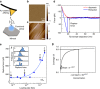
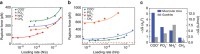
Long residence times of soil organic matter have been attributed to reactive mineral surface sites that sorb organic species and cause inaccessibility due to physical isolation and chemical stabilization at the organic-mineral interface. Instrumentation for probing this interface is limited. As a result, much of the micron- and molecular-scale knowledge about organic-mineral interactions remains largely qualitative. Here we report the use of force spectroscopy to directly measure the binding between organic ligands with known chemical functionalities and soil minerals in aqueous environments. By systematically studying the role of organic functional group chemistry with model minerals, we demonstrate that chemistry of both the organic ligand and mineral contribute to values of binding free energy and that changes in pH and ionic strength produce significant differences in binding energies. These direct measurements of molecular binding provide mechanistic insights into organo-mineral interactions, which could potentially inform land-carbon models that explicitly include mineral-bound C pools.Most molecular scale knowledge on soil organo-mineral interactions remains qualitative due to instrument limitations. Here, the authors use force spectroscopy to directly measure free binding energy between organic ligands and minerals and find that both chemistry and environmental conditions affect binding.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Kleber M, Sollins P, Sutton R. A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry. 2007;85:9–24. doi: 10.1007/s10533-007-9103-5. - DOI
-
- Kleber M, et al. Mineral-organic associations: formation, properties, and relevance in soil environments. Adv. Agron. 2015;130:1–140. doi: 10.1016/bs.agron.2014.10.005. - DOI
-
- Doetterl S, et al. Soil carbon storage controlled by interactions between geochemistry and climate. Nat. Geosci. 2015;8:780–783. doi: 10.1038/ngeo2516. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

