Dual roles of Aβ in proliferative processes in an amyloidogenic model of Alzheimer's disease
- PMID: 28855626
- PMCID: PMC5577311
- DOI: 10.1038/s41598-017-10353-7
Dual roles of Aβ in proliferative processes in an amyloidogenic model of Alzheimer's disease
Abstract
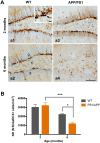
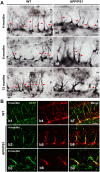
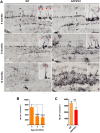
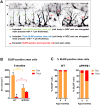
Alzheimer's disease is a major neurodegenerative disorder that leads to severe cognitive deficits in the elderly population. Over the past two decades, multiple studies have focused on elucidating the causative factors underlying memory defects in Alzheimer's patients. In this regard, new evidence linking Alzheimer's disease-related pathology and neuronal stem cells suggests that hippocampal neurogenesis impairment is an important factor underlying these cognitive deficits. However, because of conflicting results, the impact of Aβ pathology on neurogenesis/gliogenesis remains unclear. Here, we investigated the effect of Aβ on neuronal and glial proliferation by using an APP/PS1 transgenic model and in vitro assays. Specifically, we showed that neurogenesis is affected early in the APP/PS1 hippocampus, as evidenced by a significant decrease in the proliferative activity due to a reduced number of both radial glia-like neural stem cells (type-1 cells) and intermediate progenitor cells (type-2 cells). Moreover, we demonstrated that soluble Aβ from APP/PS1 mice impairs neuronal cell proliferation using neurosphere cultures. On the other hand, we showed that oligomeric Aβ stimulates microglial proliferation, whereas no effect was observed on astrocytes. These findings indicate that Aβ has a differential effect on hippocampal proliferative cells by inhibiting neuronal proliferation and triggering the formation of microglial cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

