Dysfunction of the circadian transcriptional factor CLOCK in mice resists chemical carcinogen-induced tumorigenesis
- PMID: 28855649
- PMCID: PMC5577256
- DOI: 10.1038/s41598-017-10599-1
Dysfunction of the circadian transcriptional factor CLOCK in mice resists chemical carcinogen-induced tumorigenesis
Abstract
The chronic disruption of circadian rhythms has been implicated in the risk of cancer development in humans and laboratory animals. The gene product CLOCK is a core molecular component of the circadian oscillator, so that mice with a mutated Clock gene (Clk/Clk) exhibit abnormal rhythms in various physiological processes. However, we demonstrated here that Clk/Clk mice resisted chemical carcinogen-induced tumorigenesis by suppressing epidermal growth factor (EGF) receptor-mediated proliferation signals. The repetitive application of 7,12-dimethylbenz[α]anthracene (DMBA) to skin on the back resulted in the significant development of tumors in wild-type mice, whereas chemically-induced tumorigenesis was alleviated in Clk/Clk mice. Although the degree of DMBA-induced DNA damage was not significantly different between wild-type and Clk/Clk mice, EGF receptor-mediated Ras activation was not detected in DMBA-treated Clk/Clk mice. Genetic and biochemical experiments revealed that the suppression of EGF receptor-mediated signal transduction in DMBA-treated Clk/Clk mice was associated with the expression of the cellular senescence factor p16INK4a. These results suggest an uncovered role for CLOCK in the development of chemical carcinogen-induced primary tumors and offers new preventive strategies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
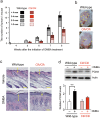


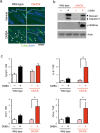
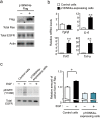
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

