Histological and ultrastructural alterations of rat thyroid gland after short-term treatment with high doses of thyroid hormones
- PMID: 28855802
- PMCID: PMC5562382
- DOI: 10.1016/j.sjbs.2015.05.006
Histological and ultrastructural alterations of rat thyroid gland after short-term treatment with high doses of thyroid hormones
Abstract
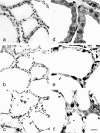
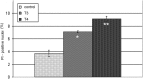
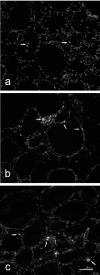
The aim of the present study was to investigate histological alterations of rat thyroid gland after short-term treatment with supraphysiological doses of thyroid hormones. Rats from experimental groups were treated with triiodothyronine (T3) or thyroxine (T4) during five days. In both treated groups, thyrocyte height was reduced and follicular lumens were distended. Progressive involutive changes of the thyroid parenchyma were apparent, including follicular remodeling (fusion) and death of thyrocytes. Morphological changes confirmed by quantitative analysis were more pronounced in the T4-treated group. Our results demonstrate that thyrotoxicosis, whether induced by T3 or T4, leads to different grades of thyroid tissue injury, including some irreversible damages. These changes might be explained at least in part by lack of trophic and cytoprotective effects of the thyroid stimulating hormone. Since the period required for morphophysiological recovery may be unpredictable, findings presented here should be taken into consideration in cases where the thyroid hormones are used as a treatment for thyroid and non-thyroid related conditions.
Keywords: Electron microscopy; Light microscopy; PI, propidium iodide; T3, triiodothyronine; T4, thyroxine; TRH, TSH-releasing hormone; TSH, thyroid stimulating hormone; Thyroid gland; Thyroid hormones; Wistar rats.
Figures



References
-
- Bauer M., Berghöfer A., Bschor T., Baumgartner A., Kiesslinger U., Hellweg R., Adli M., Baethge C., Müller-Oerlinghausen B. Supraphysiological doses of l-thyroxine in the maintenance treatment of prophylaxis-resistant affective disorders. Neuropsychopharmacology. 2002;27:620–628. - PubMed
-
- Belchetz P.E., Gredley G., Bird D., Himsworth R.L. Regulation of thyrotropin secretion by negative feedback of tri-iodothyronine on the hypothalamus. J. Endocrinol. 1978;76:439–448. - PubMed
-
- Berstein L.M. The effect of physiological doses of thyroxine on the level of cyclic adenosine 3′,5′- monophosphate in pituitary and anterior hypothalamus of male rats of different age. Endocrinologie. 1980;75:29–34. - PubMed
-
- Boelaert K., Franklyn J.A. Thyroid hormone in health and disease. J. Endocrinol. 2005;187:1–15. - PubMed
-
- Brabant G. Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J. Clin. Endocrinol. Metab. 2008;93:1167–1169. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

