Interactions between Intestinal Microbiota and Host Immune Response in Inflammatory Bowel Disease
- PMID: 28855901
- PMCID: PMC5558048
- DOI: 10.3389/fimmu.2017.00942
Interactions between Intestinal Microbiota and Host Immune Response in Inflammatory Bowel Disease
Abstract
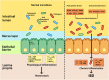
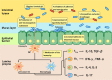
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract. Although the etiology and pathogenesis of IBD remain unclear, both genetic susceptibility and environmental factors are implicated in the initiation and progression of IBD. Recent studies with experimental animal models and clinical patients indicated that the intestinal microbiota is one of the critical environmental factors that influence nutrient metabolism, immune responses, and the health of the host in various intestinal diseases, including ulcerative colitis and Crohn's disease. The objective of this review is to highlight the crosstalk between gut microbiota and host immune response and the contribution of this interaction to the pathogenesis of IBD. In addition, potential therapeutic strategies targeting the intestinal micro-ecosystem in IBD are discussed.
Keywords: epithelial cells; host immune response; inflammatory bowel disease; intestinal barrier function; intestinal microbiota.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

