Optical coherence tomography angiography of retinal vascular occlusions produced by imaging-guided laser photocoagulation
- PMID: 28856036
- PMCID: PMC5560826
- DOI: 10.1364/BOE.8.003571
Optical coherence tomography angiography of retinal vascular occlusions produced by imaging-guided laser photocoagulation
Abstract
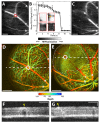
Retinal vascular occlusive diseases represent a major form of vision loss worldwide. Rodent models of these diseases have traditionally relied upon a slit-lamp biomicroscope to help visualize the fundus and subsequently aid delivery of high-power laser shots to a target vessel. Here we describe a multimodal imaging system that can produce, image, and monitor retinal vascular occlusions in rodents. The system combines a spectral-domain optical coherence tomography system for cross-sectional structural imaging and three-dimensional angiography, and a fluorescence scanning laser ophthalmoscope for Rose Bengal monitoring and high-power laser delivery to a target vessel. This multimodal system facilitates the precise production of occlusions in the branched retinal veins, central retinal vein, and branched retinal arteries. Additionally, changes in the retinal morphology and retinal vasculature can be longitudinally documented. With our device, retinal vascular occlusions can be easily and consistently created, which paves the way for futures studies on their pathophysiology and therapeutic targets.
Keywords: (170.0110) Imaging systems; (170.2520) Fluorescence microscopy; (170.4470) Ophthalmology; (170.4500) Optical coherence tomography.
Figures





References
-
- Rogers S., McIntosh R. L., Cheung N., Lim L., Wang J. J., Mitchell P., Kowalski J. W., Nguyen H., Wong T. Y., International Eye Disease Consortium , “The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia,” Ophthalmology 117, 313–319 (2010). - PMC - PubMed
-
- Rehak M., Hollborn M., Iandiev I., Pannicke T., Karl A., Wurm A., Kohen L., Reichenbach A., Wiedemann P., Bringmann A., “Retinal gene expression and Müller cell responses after branch retinal vein occlusion in the rat,” Invest. Ophthalmol. Vis. Sci. 50(5), 2359–2367 (2009).10.1167/iovs.08-2332 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
