Label-free optical detection of action potential in mammalian neurons
- PMID: 28856044
- PMCID: PMC5560835
- DOI: 10.1364/BOE.8.003700
Label-free optical detection of action potential in mammalian neurons
Abstract
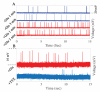
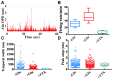
We describe an optical technique for label-free detection of the action potential in cultured mammalian neurons. Induced morphological changes due to action potential propagation in neurons are optically interrogated with a phase sensitive interferometric technique. Optical recordings composed of signal pulses mirror the electrical spike train activity of individual neurons in a network. The optical pulses are transient nanoscale oscillatory changes in the optical path length of varying peak magnitude and temporal width. Exogenous application of glutamate to cortical neuronal cultures produced coincident increase in the electrical and optical activity; both were blocked by application of a Na-channel blocker, Tetrodotoxin. The observed transient change in optical path length in a single optical pulse is primarily due to physical fluctuations of the neuronal cell membrane mediated by a yet unknown electromechanical transduction phenomenon. Our analysis suggests a traveling surface wave in the neuronal cell membrane is responsible for the measured optical signal pulses.
Keywords: (120.3180) Interferometry; (120.5050) Phase measurement; (120.5820) Scattering measurements; (170.2655) Functional monitoring and imaging; (180.3170) Interference microscopy.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
