Prevalence of HCV genotypes in Poland - the EpiTer study
- PMID: 28856279
- PMCID: PMC5497426
- DOI: 10.5114/ceh.2016.63871
Prevalence of HCV genotypes in Poland - the EpiTer study
Abstract
The aim of the study: Was to assess current prevalence of hepatitis C virus (HCV) genotypes in Poland, including their geographic distribution and changes in a given period of time.
Material and methods: Data were collected with questionnaires from 29 Polish centers and included data of patients diagnosed with HCV infection between 1 January 2013 and 31 March 2016.
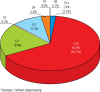
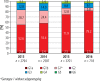
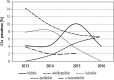
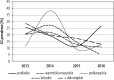
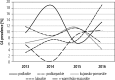
Results: In total, data of 9800 patients were reported. The highest prevalence was estimated for genotype 1b (81.7%), followed by 3 (11.3%), 4 (3.5%), 1a (3.2%) and 2 (0.2%). Genotype 5 or 6 was reported in 6 patients only (0.1%). The highest prevalence of genotype 1 was observed in central (łódzkie, mazowieckie, świętokrzyskie), eastern (lubelskie) and southern (małopolskie, śląskie) Poland. The highest rate for genotype 3 was observed in south-western (dolnośląskie, lubuskie) and eastern (podlaskie, warmińsko-mazurskie and podkarpackie) Poland. Compared to historical data, we observed an increasing tendency of G1 prevalence from 72.0% in 2003 to 87.5% in 2016, which was accompanied by a decrease of G3 (17.9% vs. 9.1%) and G4 (9.0% vs. 3.1%).
Conclusions: Almost 85% of patients with HCV in Poland are infected with genotype 1 (almost exclusively subgenotype 1b), and its prevalence shows an increasing tendency, accompanied by a decrease of genotypes 3 and 4.
Keywords: epidemiology; hepatitis C; infection; liver.
Figures
References
-
- Flisiak R, Halota W, Horban A, et al. Prevalence and risk factors of HCV infection in Poland. Eur J Gastroenterol Hepatol. 2011;23:1213–1217. - PubMed
-
- Flisiak R, Halota W, Tomasiewicz K, et al. Forecasting the disease burden of chronic hepatitis C virus in Poland. Eur J Gastroenterol Hepatol. 2015;27:70–76. - PubMed
-
- Jaroszewicz J, Flisiak R, Dusheiko G. A pill for HCV – myth or foreseeable future? Liver Int. 2014;34:6–11. - PubMed
-
- Jaroszewicz J, Flisiak-Jackiewicz M, Lebensztejn D, et al. Current drugs in early development for treating hepatitis C virus-related hepatic fibrosis. Expert Opin Investig Drugs. 2015;24:1229–1239. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
