Methamphetamine-Induced Brain Injury and Alcohol Drinking
- PMID: 28856500
- PMCID: PMC5795265
- DOI: 10.1007/s11481-017-9764-3
Methamphetamine-Induced Brain Injury and Alcohol Drinking
Abstract
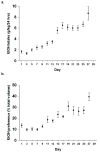
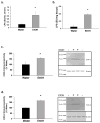
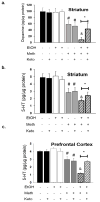
A majority of methamphetamine (Meth) abusers also abuse alcohol but the neurochemical consequences of this co-abuse are unknown. Individually, alcohol and Meth cause inflammation and long-term alterations in dopamine and serotonin signaling within the brain. Experiments were conducted to identify if serial exposure to alcohol and Meth has neurochemical consequences that are greater than after either drug alone. Male Sprague Dawley rats voluntarily drank 10% ethanol (EtOH) every other day for 4 weeks and were then exposed to a binge injection regimen of Meth (10 mg/kg injected every 2 h, for a total of 4 injections). EtOH drinking and preference increased over the 4 weeks and caused inflammation evidenced by increases in serum and brain lipopolysaccharide (LPS) and brain cyclooxygenase-2 (COX-2) 24 h after the last day of drinking. Meth alone depleted dopamine and serotonin in the striatum, as well as serotonin in the prefrontal cortex when measured 1 week later. In contrast, EtOH drinking alone did not affect dopamine and serotonin content in the striatum and prefrontal cortex, but prior EtOH drinking followed by injections of Meth enhanced Meth-induced depletions of dopamine, serotonin, as well as dopamine and serotonin transporter immunoreactivities in a manner that was correlated with the degree of EtOH consumption. Cyclooxygenase inhibition by ketoprofen during EtOH drinking blocked the increases in LPS and COX-2 and the enhanced decreases in dopamine and serotonin produced by Meth. Therefore, prior EtOH drinking causes an increase in inflammatory mediators that mediate a synergistic interaction with Meth to cause an enhanced neurotoxicity.
Keywords: Alcohol; Cyclooxygenase-2; Lipopolysaccharide; Methamphetamine; Monoamines.
Figures





References
-
- Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N. Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neuroscience letters. 2003;352:13–16. - PubMed
-
- Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Current Pharmaceutical Design. 2005;11:973–984. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

