Safety of Antiplatelet Agents: Analysis of 'Real-World' Data from the Italian National Pharmacovigilance Network
- PMID: 28856572
- PMCID: PMC5643364
- DOI: 10.1007/s40261-017-0566-4
Safety of Antiplatelet Agents: Analysis of 'Real-World' Data from the Italian National Pharmacovigilance Network
Abstract
Introduction: According to the Italian National Report on drug use, thienopyridines (ticlopidine, clopidogrel and prasugrel) and ticagrelor represent the most prescribed antiplatelet agents, beside aspirin. The aim of this study was to analyse the safety profile of these drugs using data from spontaneous reporting of suspected adverse reactions (ADRs).
Methods: Suspected ADRs for ticlopidine, clopidogrel, prasugrel and ticagrelor, reported on the Italian National Pharmacovigilance Network between January 2009 and December 2016, were included in the analysis. All suspected ADRs were classified by frequency, seriousness, outcome, age and system organ class.
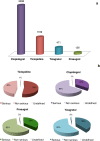
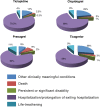
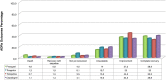
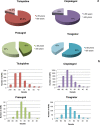
Results: Clopidogrel showed the highest absolute number of suspected ADRs, followed by ticlopidine. However, these data need to be contextualized in view of the differences in marketing authorization dates, prescription rates and a characterization of the relative seriousness of ADRs per each drug. After the correction for prescription rate, ticagrelor showed the highest reporting trend and ticlopidine the lowest. Most ADRs occurred in the elderly, in particular for ticlopidine. Bleeding represents one of the most reported events (ticlopidine 40%, clopidogrel 26%, prasugrel 42%, ticagrelor 30%) and aspirin was the most frequently associated suspected drug. The majority of ADRs had complete recovery and were non-serious, except for ticlopidine (serious ADRs 53%). Prasugrel showed the highest percentage of 'life-threatening' events and 'death'.
Conclusions: Based on the analysis conducted on spontaneous ADRs reporting system in Italy, the safety profile of antiplatelet drugs seems favourable. However, the overall risk-benefit ratio of these drugs needs to be reassessed taking into account the appropriateness of use in particular populations at risk, such as the elderly. Based on this information, we believe that more attention from clinicians and/or an implementation of regulatory measures could be useful for clinical practice.
Conflict of interest statement
Funding
No funding was received for the production of this paper.
Conflict of interest
Authors declare that they have no conflicts of interest.
Figures
References
-
- Agenzia Italiana del Farmaco—L’uso dei Farmaci in Italia. Rapporto OSMED 2015. June 2016.
-
- Task Force M, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

