Whole blood microRNA markers are associated with acute respiratory distress syndrome
- PMID: 28856588
- PMCID: PMC5577350
- DOI: 10.1186/s40635-017-0155-0
Whole blood microRNA markers are associated with acute respiratory distress syndrome
Abstract
Background: MicroRNAs (miRNAs) can play important roles in inflammation and infection, which are common manifestations of acute respiratory distress syndrome (ARDS). We assessed if whole blood miRNAs were potential diagnostic biomarkers for human ARDS.
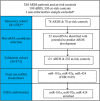
Methods: This nested case-control study (N = 530) examined a cohort of ARDS patients and critically ill at-risk controls. Whole blood miRNA profiles and logistic regression analyses identified miRNAs correlated with ARDS. Stratification analysis also assessed selected miRNA markers for their role in sepsis and pneumonia associated with ARDS. Receiver operating characteristic (ROC) analysis evaluated miRNA diagnostic performance, along with Lung Injury Prediction Score (LIPS).
Results: Statistical analyses were performed on 294 miRNAs, selected from 754 miRNAs after quality control screening. Logistic regression identified 22 miRNAs from a 156-patient discovery cohort as potential risk or protective markers of ARDS. Three miRNAs-miR-181a, miR-92a, and miR-424-from the discovery cohort remained significantly associated with ARDS in a 373-patient independent validation cohort (FDR q < 0.05) and meta-analysis (p < 0.001). ROC analyses demonstrated a LIPS baseline area-under-the-curve (AUC) value of ARDS of 0.708 (95% CI 0.651-0.766). Addition of miR-181a, miR-92a, and miR-424 to LIPS increased baseline AUC to 0.723 (95% CI 0.667-0.778), with a relative integrated discrimination improvement of 2.40 (p = 0.005) and a category-free net reclassification index of 27.21% (p = 0.01).
Conclusions: miR-181a and miR-92a are risk biomarkers for ARDS, whereas miR-424 is a protective biomarker. Addition of these miRNAs to LIPS can improve the risk estimate for ARDS.
Keywords: ARDS; LIPS; MicroRNA; Whole blood.
Conflict of interest statement
Ethics approval and consent to participate
The institutional review boards of the MGH, BIDMC, and Harvard T.H. Chan School of Public Health approved this study.
Consent for publication
We confirm that we have obtained consent from participants (or legal parent/guardian for children) to report individual patient data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, DF MA, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, Group ET Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA : the journal of the American Medical Association. 2016;315:788–800. doi: 10.1001/jama.2016.0291. - DOI - PubMed
-
- Zhu Z, Wang T, Liu Z, Yi L, Yang Z, Bian W, Chen W, Wang S, Li G, Li A, Martin GS, Zhu X. Plasma neutrophil elastase and elafin as prognostic biomarker for acute respiratory distress syndrome: a multicenter survival and longitudinal prospective observation study. Shock. 2017;48(2):168–174. doi: 10.1097/SHK.0000000000000845. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources