The use of dimethylsulfoxide as a solvent in enzyme inhibition studies: the case of aldose reductase
- PMID: 28856935
- PMCID: PMC6009938
- DOI: 10.1080/14756366.2017.1363744
The use of dimethylsulfoxide as a solvent in enzyme inhibition studies: the case of aldose reductase
Abstract
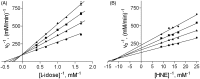
Aldose reductase (AR) is an enzyme devoted to cell detoxification and at the same time is strongly involved in the aetiology of secondary diabetic complications and the amplification of inflammatory phenomena. AR is subjected to intense inhibition studies and dimethyl sulfoxide (DMSO) is often present in the assay mixture to keep the inhibitors in solution. DMSO was revealed to act as a weak but well detectable AR differential inhibitor, acting as a competitive inhibitor of the L-idose reduction, as a mixed type of non-competitive inhibitor of HNE reduction and being inactive towards 3-glutathionyl-4-hydroxynonanal transformation. A kinetic model of DMSO action with respect to differently acting inhibitors was analysed. Three AR inhibitors, namely the flavonoids neohesperidin dihydrochalcone, rutin and phloretin, were used to evaluate the effects of DMSO on the inhibition studies on the reduction of L-idose and HNE.
Keywords: Dimethyl sulfoxide; aldose reductase; aldose reductase differential inhibitors.
Figures

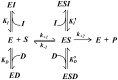

References
-
- Saytzeff A. “Ueber die Einwirkung von Saltpetersäure auf Schwefelmethyl und Schwefeläthyl” (On the effect of nitric acid on methyl sulfide and ethyl sulfide). Ann Der Chem Und Pharm 1867;144:148–56.
-
- Rammler DH, Zaffaroni A.. Biological implications of DMSO based on a review of its chemical properties. Ann N Y Acad Sci 1967;141:13–23. - PubMed
-
- Romanowski SJ, Kinart CM, Kinart WJ.. Physicochemical properties of dimethyl sulfoxide–methanol liquid mixtures. Experimental and semiempirical quantum chemical studies. J Chem Soc Faraday Trans 1995;91:65–70.
-
- Epstein WW, Sweat FW.. Dimethyl sulfoxide oxidations. Chem Rev 1967;67:247–60. - PubMed
-
- David NA. The Pharmacology of dimethyl sulfoxide. Annu Rev Pharmacol 1972;12:353–74. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
