The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain
- PMID: 28857069
- PMCID: PMC5719110
- DOI: 10.1038/npp.2017.204
The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain
Abstract
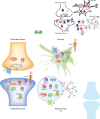
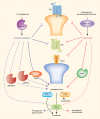
A great need exists for the development of new medications to treat pain resulting from various disease states and types of injury. Given that the endogenous cannabinoid (that is, endocannabinoid) system modulates neuronal and immune cell function, both of which play key roles in pain, therapeutics targeting this system hold promise as novel analgesics. Potential therapeutic targets include the cannabinoid receptors, type 1 and 2, as well as biosynthetic and catabolic enzymes of the endocannabinoids N-arachidonoylethanolamine and 2-arachidonoylglycerol. Notably, cannabinoid receptor agonists as well as inhibitors of endocannabinoid-regulating enzymes fatty acid amide hydrolase and monoacylglycerol lipase produce reliable antinociceptive effects, and offer opioid-sparing antinociceptive effects in myriad preclinical inflammatory and neuropathic pain models. Emerging clinical studies show that 'medicinal' cannabis or cannabinoid-based medications relieve pain in human diseases such as cancer, multiple sclerosis, and fibromyalgia. However, clinical data have yet to demonstrate the analgesic efficacy of inhibitors of endocannabinoid-regulating enzymes. Likewise, the question of whether pharmacotherapies aimed at the endocannabinoid system promote opioid-sparing effects in the treatment of pain reflects an important area of research. Here we examine the preclinical and clinical evidence of various endocannabinoid system targets as potential therapeutic strategies for inflammatory and neuropathic pain conditions.
Figures
References
-
- Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT et al (2011). Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther 338: 114–124. - PMC - PubMed
-
- Aley KO, Reichling DB, Levine JD (1996). Vincristine hyperalgesia in the rat: a model of painful vincristine neuropathy in humans. Neuroscience 73: 259–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

