Chimeric antigen receptor T-cell therapies for lymphoma
- PMID: 28857075
- PMCID: PMC12145160
- DOI: 10.1038/nrclinonc.2017.128
Chimeric antigen receptor T-cell therapies for lymphoma
Abstract
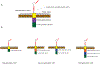
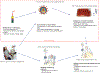
New therapies are needed for patients with Hodgkin or non-Hodgkin lymphomas that are resistant to standard therapies. Indeed, unresponsiveness to standard chemotherapy and relapse after autologous stem-cell transplantation are indicators of an especially poor prognosis. Chimeric antigen receptor (CAR) T cells are emerging as a novel treatment modality for these patients. Clinical trial data have demonstrated the potent activity of anti-CD19 CAR T cells against multiple subtypes of B-cell lymphoma, including diffuse large-B-cell lymphoma (DLBCL), follicular lymphoma, mantle-cell lymphoma, and marginal-zone lymphoma. Importantly, anti-CD19 CAR T cells have impressive activity against chemotherapy-refractory lymphoma, inducing durable complete remissions lasting >2 years in some patients with refractory DLBCL. CAR-T-cell therapies are, however, associated with potentially fatal toxicities, including cytokine-release syndrome and neurological toxicities. CAR T cells with novel target antigens, including CD20, CD22, and κ-light chain for B-cell lymphomas, and CD30 for Hodgkin and T-cell lymphomas, are currently being investigated in clinical trials. Centrally manufactured CAR T cells are also being tested in industry-sponsored multicentre clinical trials, and will probably soon become a standard therapy. Herein, we review the clinical efficacy and toxicity of CAR-T-cell therapies for lymphoma, and discuss their limitations and future directions with regard to toxicity management, CAR designs and CAR-T-cell phenotypes, conditioning regimens, and combination therapies.
Figures
References
-
- “About Non-Hodgkin Lymphoma.” American Cancer Society website. 2017. https://www.cancer.org/cancer/non-hodgkin-lymphoma/about/key-statistics..... .
-
- Coiffier B et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346, 235–42 (2002). - PubMed
-
- Marcus R et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 26, 4579–86 (2008). - PubMed
-
- Forstpointner R et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 104, 3064–71 (2004). - PubMed
-
- Elstrom RL et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk 10, 192–6 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

