Elevated resting H+ current in the R1239H type 1 hypokalaemic periodic paralysis mutated Ca2+ channel
- PMID: 28857175
- PMCID: PMC5638893
- DOI: 10.1113/JP274638
Elevated resting H+ current in the R1239H type 1 hypokalaemic periodic paralysis mutated Ca2+ channel
Abstract
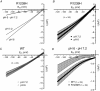
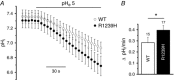
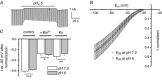
Key points: Missense mutations in the gene encoding the α1 subunit of the skeletal muscle voltage-gated Ca2+ channel induce type 1 hypokalaemic periodic paralysis, a poorly understood neuromuscular disease characterized by episodic attacks of paralysis associated with low serum K+ . Acute expression of human wild-type and R1239H HypoPP1 mutant α1 subunits in mature mouse muscles showed that R1239H fibres displayed Ca2+ currents of reduced amplitude and larger resting leak inward current increased by external acidification. External acidification also produced intracellular acidification at a higher rate in R1239H fibres and inhibited inward rectifier K+ currents. These data suggest that the R1239H mutation induces an elevated leak H+ current at rest flowing through a gating pore and could explain why paralytic attacks preferentially occur during the recovery period following muscle exercise.
Abstract: Missense mutations in the gene encoding the α1 subunit of the skeletal muscle voltage-gated Ca2+ channel induce type 1 hypokalaemic periodic paralysis, a poorly understood neuromuscular disease characterized by episodic attacks of paralysis associated with low serum K+ . The present study aimed at identifying the changes in muscle fibre electrical properties induced by acute expression of the R1239H hypokalaemic periodic paralysis human mutant α1 subunit of Ca2+ channels in a mature muscle environment to better understand the pathophysiological mechanisms involved in this disorder. We transferred genes encoding wild-type and R1239H mutant human Ca2+ channels into hindlimb mouse muscle by electroporation and combined voltage-clamp and intracellular pH measurements on enzymatically dissociated single muscle fibres. As compared to fibres expressing wild-type α1 subunits, R1239H mutant-expressing fibres displayed Ca2+ currents of reduced amplitude and a higher resting leak inward current that was increased by external acidification. External acidification also produced intracellular acidification at a higher rate in R1239H fibres and inhibited inward rectifier K+ currents. These data indicate that the R1239H mutation induces an elevated leak H+ current at rest flowing through a gating pore created by the mutation and that external acidification favours onset of muscle paralysis by potentiating H+ depolarizing currents and inhibiting resting inward rectifier K+ currents. Our results could thus explain why paralytic attacks preferentially occur during the recovery period following intense muscle exercise.
Keywords: gating pore; myopathy; voltage-gated Ca2+ channel.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures





Comment in
-
An atypical CaV1.1 mutation reveals a common mechanism for hypokalemic periodic paralysis.J Gen Physiol. 2017 Dec 4;149(12):1061-1064. doi: 10.1085/jgp.201711923. Epub 2017 Nov 14. J Gen Physiol. 2017. PMID: 29138267 Free PMC article.
References
-
- Allsop P, Cheetham M, Brooks S, Hall GM & Williams C (1990). Continuous intramuscular pH measurement during the recovery from brief, maximal exercise in man. Eur J Appl Physiol 59, 465–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

