Effect of direct cold atmospheric plasma (diCAP) on microcirculation of intact skin in a controlled mechanical environment
- PMID: 28857373
- PMCID: PMC6084368
- DOI: 10.1111/micc.12399
Effect of direct cold atmospheric plasma (diCAP) on microcirculation of intact skin in a controlled mechanical environment
Abstract
Objective: The microcirculatory response of intact human skin to exposure with diCAP for different durations with a focus on the effect of implied mechanical pressure during plasma treatment was investigated.
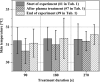
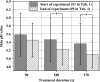
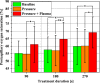
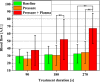
Methods: Local relative hemoglobin, blood flow velocity, tissue oxygen saturation, and blood flow were monitored noninvasively for up to 1 hour in 1-2 mm depth by optical techniques, as well as temperature, pH values, and moisture before and after skin stimulation. The experimental protocol (N = 10) was set up to differentiate between pressure- and plasma-induced effects.
Results: Significant increases in microcirculation were only observed after plasma stimulation but not after pressure stimulus alone. For a period of 1 h after stimulation, local relative hemoglobin was increased by 5.1% after 270 seconds diCAP treatment. Tissue oxygen saturation increased by up to 9.4%, whereas blood flow was doubled (+106%). Skin pH decreased by 0.3 after 180 seconds and 270 seconds diCAP treatment, whereas skin temperature and moisture were not affected.
Conclusions: diCAP treatment of intact skin notably enhances microcirculation for a therapeutically relevant period. This effect is specific to the plasma treatment and not an effect of the applied pressure. Prolonged treatment durations lead to more pronounced effects.
Keywords: blood flow; cold atmospheric plasma; hemoglobin; microcirculation; plasma medicine; skin; tissue oxygen saturation; transcutaneous oxygen pressure; wound healing; wounds.
© 2017 John Wiley & Sons Ltd.
Figures





References
-
- d′Agostino R, Favia P, Oehr C, et al. Low‐temperature plasma processing of materials: past, present, and future. Plasma Processes Polym. 2005;2:7‐15.
-
- Stoffels E, Flikweert AJ, Stoffels WW, et al. Plasma needle: a non‐destructive atmospheric plasma source for fine surface treatment of (bio)materials. Plasma Sources Sci Technol. 2002;11:383‐388.
-
- Tuemmel S, Mertens N, Wang J, et al. Low temperature plasma treatment of living human cells. Plasma Processes Polym. 2007;4:465‐469.
-
- Kuchenbecker M, Bibinov N, Kaemling A, et al. Characterization of DBD plasma source for biomedical applications. J Phys D Appl Phys. 2009;42:45212(10 pp).
-
- Weltmann KD, Kindel E, Brandenburg R, et al. Atmospheric pressure plasma jet for medical therapy: plasma parameters and risk estimation. Contrib Plasma Phys. 2009;49:631‐640.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

