Vinblastine 20' Amides: Synthetic Analogues That Maintain or Improve Potency and Simultaneously Overcome Pgp-Derived Efflux and Resistance
- PMID: 28857558
- PMCID: PMC5599373
- DOI: 10.1021/acs.jmedchem.7b00958
Vinblastine 20' Amides: Synthetic Analogues That Maintain or Improve Potency and Simultaneously Overcome Pgp-Derived Efflux and Resistance
Abstract
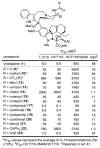
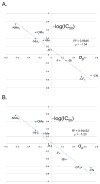
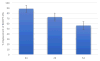
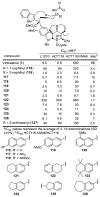
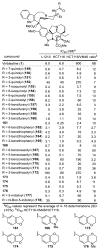
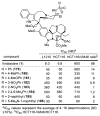
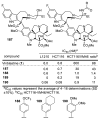
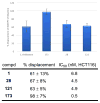
A series of 180 vinblastine 20' amides were prepared in three steps from commercially available starting materials, systematically exploring a typically inaccessible site in the molecule enlisting a powerful functionalization strategy. Clear structure-activity relationships and a structural model were developed in the studies which provided many such 20' amides that exhibit substantial and some even remarkable enhancements in potency, many that exhibit further improvements in activity against a Pgp overexpressing resistant cancer cell line, and an important subset of the vinblastine analogues that display little or no differential in activity against a matched pair of vinblastine sensitive and resistant (Pgp overexpressing) cell lines. The improvements in potency directly correlated with target tubulin binding affinity, and the reduction in differential functional activity against the sensitive and Pgp overexpressing resistant cell lines was found to correlate directly with an impact on Pgp-derived efflux.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








References
-
- Neuss N, Neuss MN. Therapeutic use of bisindole alkaloids from catharanthus. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. Academic; San Diego, CA: 1990. pp. 229–240.
-
- Pearce HL. Medicinal chemistry of bisindole alkaloids from Catharanthus. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. Academic; San Diego, CA: 1990. pp. 145–204.
-
- Kuehne ME, Marko I. Syntheses of vinblastine-type alkaloids. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. Academic; San Diego, CA: 1990. pp. 77–132.
-
- Noble RL, Beer CT, Cutts JH. Role of chance observations in chemotherapy: Vinca rosea. Ann NY Acad Sci. 1958;76:882–894. - PubMed
-
- Svoboda GH, Nuess N, Gorman M. Alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don.). V. Preparation and characterization of alkaloids. J Am Pharm Assoc Sci Ed. 1959;48:659–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

