Barbed Ribbon Device for Progressive Tension Closure Reduces Seroma After Breast Reconstruction
- PMID: 28857777
- PMCID: PMC5690306
- DOI: 10.1097/SAP.0000000000001217
Barbed Ribbon Device for Progressive Tension Closure Reduces Seroma After Breast Reconstruction
Abstract
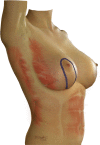
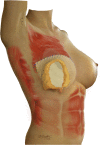
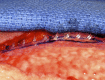
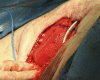
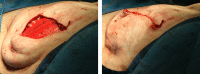
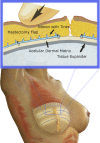
Purpose: Implant-based breast reconstruction is fraught with complications related to seroma formation. Soft tissue stabilization with progressive tension closure (PTC) has been shown to decrease seroma formation after various procedures but is less suitable for mastectomy flap stabilization. We evaluate the incidence of seroma in breast reconstruction using bioabsorbable barbed ribbon devices (BRDs) as a novel approach to PTC.
Methods: We performed a retrospective review of all patients whose mastectomy flaps were stabilized with BRDs. These patients were compared with consecutive patients who underwent mastectomy and reconstruction without progressive tension flap stabilization. Patient demographics and outcomes were recorded, including comorbidities, complications, presence of seroma, and total drain days.
Results: In the BRD-PTC group, there were 36 breasts compared with 56 in the nonstabilized control group. There were no significant differences in rate of tobacco use, age, or body mass index. We identified 11 seromas in the control group (19.6%) and none in the intervention group (P = 0.05). In the PTC group, drains were removed an average of 5 days sooner than those in controls (P = 0.006).
Conclusion: Progressive tension stabilization of mastectomy flaps with BRD significantly reduces seroma formation and the duration for which closed suction drainage is required.
Conflict of interest statement
Conflicts of interest and sources of funding: Drs Devan Griner, Caleb Steffen, and Kristopher Day have no financial disclosures or commercial associations that might pose or create a conflict of interest with any information presented in this article. Dr Mark Brzezienski is a Lifecell consultant for a product not discussed in the article. Lifecell (Alloderm) and MicroAire Surgical Instruments (Endotine) provided no funding or resources for the study. Funding for statistical analysis, illustrations, and travel reimbursement for presentation at SESPRS provided by the Chattanooga Plastic Surgery Foundation (Chattanooga, TN).
Figures

References
-
- Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. - PubMed
-
- Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614. - PubMed
-
- Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–678. - PubMed
-
- Chilson TR, Chan FD, Lonser RR, et al. Seroma prevention after modified radical mastectomy. Am Surg. 1992;58:750–754. - PubMed
-
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

