Stem Cell-Like Properties of CK2β-down Regulated Mammary Cells
- PMID: 28858215
- PMCID: PMC5615329
- DOI: 10.3390/cancers9090114
Stem Cell-Like Properties of CK2β-down Regulated Mammary Cells
Abstract
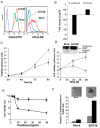
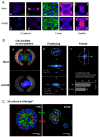
The ubiquitous protein kinase CK2 has been demonstrated to be overexpressed in a number of human tumours. This enzyme is composed of two catalytic α or α' subunits and a dimer of β regulatory subunits whose expression levels are probably implicated in CK2 regulation. Several recent papers reported that unbalanced expression of CK2 subunits is sufficient to drive epithelial to mesenchymal transition, a process involved in cancer invasion and metastasis. Herein, through transcriptomic and miRNA analysis together with comparison of cellular properties between wild type and CK2β-knock-down MCF10A cells, we show that down-regulation of CK2β subunit in mammary epithelial cells induces the acquisition of stem cell-like properties associated with perturbed polarity, CD44high/CD24low antigenic phenotype and the ability to grow under anchorage-independent conditions. These data demonstrate that a CK2β level establishes a critical cell fate threshold in the control of epithelial cell plasticity. Thus, this regulatory subunit functions as a nodal protein to maintain an epithelial phenotype and its depletion drives breast cell stemness.
Keywords: EMT; breast cancer; epithelial plasticity; protein kinase CK2; stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

