Preparation and Characterization of Novel Cationic Chitosan Derivatives Bearing Quaternary Ammonium and Phosphonium Salts and Assessment of Their Antifungal Properties
- PMID: 28858241
- PMCID: PMC6151502
- DOI: 10.3390/molecules22091438
Preparation and Characterization of Novel Cationic Chitosan Derivatives Bearing Quaternary Ammonium and Phosphonium Salts and Assessment of Their Antifungal Properties
Abstract
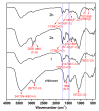
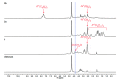
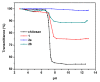
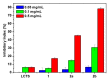
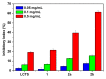
Chitosan is an abundant and renewable polysaccharide, its derivatives exhibit attractive bioactivities and the wide applications in various biomedical fields. In this paper, two novel cationic chitosan derivatives modified with quaternary phosphonium salts were successfully synthesized via trimethylation, chloride acetylation, and quaternization with tricyclohexylphosphine and triphenylphosphine. The structures and properties of synthesized products in the reactions were characterized by FTIR spectroscopy, ¹H-NMR, 31P-NMR, elemental and thermogravimetric analysis. The antifungal activities of chitosan derivatives against four kinds of phytopathogens, including Phomopsis asparagi, Watermelon fusarium, Colletotrichum lagenarium, and Fusarium oxysporum were tested using the radial growth assay in vitro. The results revealed that the synthesized cationic chitosan derivatives showed significantly improved antifungal efficiency compared to chitosan. It was reasonably suggested that quaternary phosphonium groups enabled the obviously stronger antifungal activity of the synthesized chitosans. Especially, the triphenylphosphonium-functionalized chitosan derivative inhibited the growth of Phomopsis asparagi most effectively, with inhibitory indices of about 80% at 0.5 mg/mL. Moreover, the data demonstrated that the substituted groups with stronger electron-withdrawing ability relatively possessed greater antifungal activity. The results suggest the possibility that cationic chitosan derivatives bearing quaternary phosphonium salts could be effectively employed as novel antifungal biomaterials for application in the field of agriculture.
Keywords: antifungal activity; cationic chitosan derivatives; electron-withdrawing ability; quaternary ammonium salts; quaternary phosphonium salts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
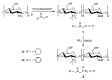




References
-
- Sajomsang W., Ruktanonchai U.R., Gonil P., Warin C. Quaternization of N-(3-pyridylmethyl) chitosan derivatives: Effects of the degree of quaternization, molecular weight and ratio of N-methylpyridinium and N,N,N-trimethyl ammonium moieties on bactericidal activity. Carbohydr. Polym. 2010;82:1143–1152. doi: 10.1016/j.carbpol.2010.06.047. - DOI
-
- Sukamporn P., Baek S.J., Gritsanapan W., Chirachanchai S., Nualsanit T., Rojanapanthu P. Self-assembled nanomicelles of damnacanthal-loaded amphiphilic modified chitosan: Preparation, characterization and cytotoxicity study. Mater. Sci. Eng. C. 2017;77:1068–1077. doi: 10.1016/j.msec.2017.03.263. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

