Enantiospecific Solvolytic Functionalization of Bromochlorides
- PMID: 28858493
- PMCID: PMC5987033
- DOI: 10.1021/jacs.7b07792
Enantiospecific Solvolytic Functionalization of Bromochlorides
Abstract
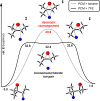
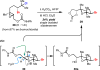
Herein, we report that under mild solvolytic conditions, enantioenriched bromochlorides can be ionized, stereospecifically cyclized to an array of complex bromocyclic scaffolds, or intermolecularly trapped by exogenous nucleophiles. Mechanistic investigations support an ionic mechanism wherein the bromochloride serves as an enantioenriched bromonium surrogate. Several natural product-relevant motifs are accessed in enantioenriched form for the first time with high levels of stereocontrol, and this technology is applied to the scalable synthesis of a polycyclic brominated natural product. Arrays of nucleophiles including olefins, alkynes, heterocycles, and epoxides are competent traps in the bromonium-induced cyclizations, leading to the formation of enantioenriched mono-, bi-, and tricyclic products. This strategy is further amenable to intermolecular coupling between cinnamyl bromochlorides and a diverse set of commercially available nucleophiles. Collectively, this work demonstrates that enantioenriched bromonium chlorides are configurationally stable under solvolytic conditions in the presence of a variety of functional groups.
Figures

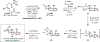
References
-
- Gribble GW. Naturally Occurring Organohalogen Compounds – A Comprehensive Survey. Springer-Verlag; Wien: 1996. - PubMed
- Gribble GW. Naturally Occurring Organohalogen Compounds – A Comprehensive Update. Springer-Verlag; Wien: 2010.
- Wang BG, Gloer JB, Ji NY, Zhao JC. Chem. Rev. 2013;113:3632–3685. - PubMed
- Brito I, Dias T, Diaz-Marrero AR, Darias J, Cueto M. Tetrahedron. 2006;62:9655–9660.
- Brito I, Dias T, Diaz-Marrero AR, Darias J, Cueto M. Tetrahedron. 2007;63:3908.
-
- Federov SN, Reshetnyak AP, Shchedrin AP, Il’in SG, Struchkov YT, Stonik VA, Elyakov GB. Dokl. Akad. Nauk SSSR. 1989;305:877–879.
- Lyakhova EG, Federov SN, Shubina LK, Radchenko OS, Kalinovsky AI, Dvitrenok PS, Stonki VA. Russ. Chem. Bull. Int. Ed. 2003;52:970–974.
- Federov SN, Shubina LK, Bode AM, Stonik VA, Dong Z. Cancer Res. 2007;67:5194–5920. - PubMed
-
- Suzuki T, Suzuki M, Furusaki A, Matsumoto T, Kato A, Imanaka Y, Kurosawa E. Tetrahedron Lett. 1985;26:1329–1332.
- Suzuki T, Takeda S, Suzuki M, Kurosawa E, Kato A, Imanaka Y. Chem. Lett. 1987:361–364.
-
- Carter-Franklin JN, Butler AJ. J. Am. Chem. Soc. 2004;126:15060–15066. - PubMed
- Fukuzawa A, Miyamoto M, Kumagai Y, Abiko A, Takaya Y, Masamune T. Chem. Lett. 1985:1259–1262.
- Findlay JA, Li G. Can. J. Chem. 2002;80:1697–1707.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

