Synaptic Mechanisms of Feature Coding in the Visual Cortex of Awake Mice
- PMID: 28858618
- PMCID: PMC5580349
- DOI: 10.1016/j.neuron.2017.08.014
Synaptic Mechanisms of Feature Coding in the Visual Cortex of Awake Mice
Abstract
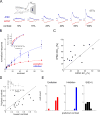
The synaptic mechanisms of feature coding in the visual cortex are poorly understood, particularly in awake animals. The ratio between excitation (E) and inhibition (I) might be constant across stimulus space, controlling only the gain and timing of neuronal responses, or it might change, directly contributing to feature coding. Whole-cell recordings in L2/3 of awake mice revealed that the E/I ratio systematically declines with increasing stimulus contrast or size. Suppressing somatostatin (SOM) neurons enhanced the E and I underlying size tuning, explaining SOM neurons' role in surround suppression. These data imply that contrast and size tuning result from a combination of a changing E/I ratio and the tuning of total synaptic input. Furthermore, they provide experimental support in awake animals for the "Stabilized Supralinear Network," a model that explains diverse cortical phenomena, and suggest that a decreasing E/I ratio with increasing cortical drive could contribute to many different cortical computations.
Keywords: E/I balance; Primary visual cortex; V1; contextual modulation; contrast sensitivity; in vivo whole cell; neural coding; normalization; optogenetics; size tuning.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures






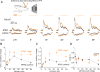
References
-
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. Journal of neurophysiology. 2000;84:909–926. - PubMed
-
- Angelucci A, Shushruth S. Beyond the Classical Receptive Field: Surround Modulation in Primary Visual Cortex. New Visual Neurosciences. 2014:425–444.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

